Here are the essential concepts you must grasp in order to answer the question correctly.
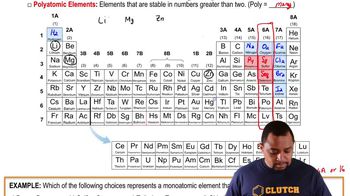
Periodic Table
The periodic table is a systematic arrangement of chemical elements, organized by increasing atomic number. Elements are grouped into rows (periods) and columns (groups) based on similar properties. Understanding the layout of the periodic table is essential for identifying elements and their classifications.
Recommended video:
Periodic Table Classifications
Element Groups
Elements in the periodic table are categorized into groups or families, which share similar chemical properties. For example, Group 1 contains alkali metals, while Group 17 includes halogens. Recognizing these groups helps predict the behavior of elements during chemical reactions.
Recommended video:
Main Group Elements: Density Example
Element Identification
Identifying elements involves recognizing their symbols and atomic numbers on the periodic table. Each element has a unique symbol (like H for hydrogen) and is placed according to its properties and reactivity. This identification is crucial for understanding their roles in chemical reactions and compounds.
Recommended video:
Chalcogen Identification Example