Here are the essential concepts you must grasp in order to answer the question correctly.
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It allows chemists to predict the amounts of substances consumed and produced in a reaction based on balanced chemical equations. Understanding stoichiometry is essential for solving problems involving mass and moles in chemical reactions.
Recommended video:
Conservation of Mass
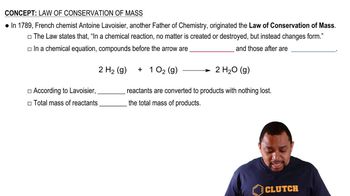
The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. This principle implies that the total mass of the reactants must equal the total mass of the products. In the context of the given reaction, this means that the mass of CaCO3 before heating must equal the combined mass of CaO and CO2 after the reaction.
Recommended video:
Law of Conservation of Mass
Chemical Reaction Equation
A chemical reaction equation represents the reactants and products involved in a chemical reaction, showing their respective quantities. For the decomposition of calcium carbonate (CaCO3), the balanced equation is: CaCO3(s) → CaO(s) + CO2(g). This equation is crucial for understanding the relationship between the substances involved and for calculating the mass of products formed from given reactants.
Recommended video:
Balancing Chemical Equations