Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Reactions
Nuclear reactions involve changes in an atom's nucleus and can result in the transformation of one element into another. These reactions include processes such as alpha decay, beta decay, and nuclear fission. Understanding the type of nuclear reaction is essential for predicting the products and balancing the equations.
Recommended video:
Conservation of Mass and Charge
In nuclear equations, both mass and charge must be conserved. This means that the total mass number (sum of protons and neutrons) and the total charge (sum of positive and negative charges) before the reaction must equal those after the reaction. Balancing these quantities is crucial for correctly completing nuclear equations.
Recommended video:
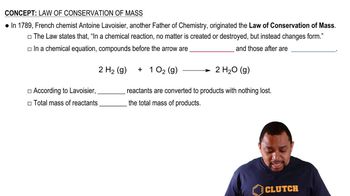
Law of Conservation of Mass
Notation in Nuclear Chemistry
Nuclear chemistry uses specific notation to represent isotopes and particles. For example, isotopes are denoted by their element symbol followed by their mass number (e.g., U-235), while particles like alpha (α) and beta (β) are represented by their symbols. Familiarity with this notation is necessary for accurately writing and balancing nuclear equations.
Recommended video:
Standard Notation to Scientific Notation
 Verified step by step guidance
Verified step by step guidance