Here are the essential concepts you must grasp in order to answer the question correctly.
Redox Reactions
Redox reactions involve the transfer of electrons between two species, where one is oxidized (loses electrons) and the other is reduced (gains electrons). In the context of the Breathalyzer, ethanol is oxidized to acetic acid, while potassium dichromate is reduced, leading to a color change in the solution. Understanding the oxidation states of the involved species is crucial for analyzing the reaction.
Recommended video:
Identifying Redox Reactions
Electrode Potential (E)
The electrode potential (E) is a measure of the tendency of a chemical species to be reduced, expressed in volts. It is determined under standard conditions but can be adjusted for concentration and pH using the Nernst equation. In this scenario, calculating E for the reaction requires knowledge of the standard reduction potentials and the concentrations of the reactants.
Recommended video:
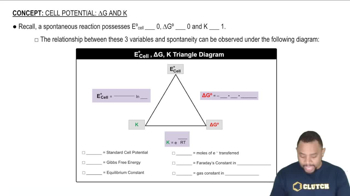
Relationship between ∆E°, ∆G°, and K
Nernst Equation
The Nernst equation relates the cell potential to the concentrations of the reactants and products at non-standard conditions. It is given by E = E° - (RT/nF)ln(Q), where E° is the standard potential, R is the gas constant, T is the temperature in Kelvin, n is the number of moles of electrons transferred, F is Faraday's constant, and Q is the reaction quotient. This equation is essential for calculating the actual electrode potential in the Breathalyzer reaction.
Recommended video: