Here are the essential concepts you must grasp in order to answer the question correctly.
Titration and pH Curves
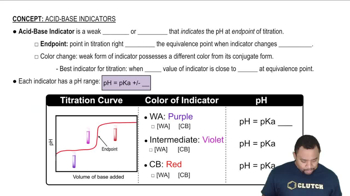
Titration is a technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. The pH curve represents the change in pH as a titrant is added, showing distinct regions where the pH changes rapidly, indicating the presence of equivalence points. These points correspond to the complete neutralization of the acid by the base, which is crucial for determining the pKa values of the acid.
Recommended video:
Acid-Base Titration Curve Example
pKa and Acid-Base Equilibria
The pKa value is a measure of the strength of an acid, defined as the negative logarithm of its acid dissociation constant (Ka). It indicates the pH at which half of the acid is dissociated, providing insight into the acid's ability to donate protons. In a titration curve, the pKa values can be identified at the inflection points where the pH changes most dramatically, corresponding to the acid's dissociation stages.
Recommended video:
Diprotic Acids
Diprotic acids are acids that can donate two protons (H+) per molecule during the titration process. They exhibit two distinct pKa values, corresponding to the two stages of dissociation. The titration curve for a diprotic acid will show two equivalence points, each associated with the loss of one proton, allowing for the determination of both pKa values from the curve.
Recommended video:
3 forms of Diprotic Acids