A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equiva-lence point was reached after 126.4 mL of base. (c) What percent of the HCl is neutralized at the first equivalence point?
A saturated solution of an ionic salt MX exhibits an osmotic pressure of 74.4 mm Hg at 25 °C. Assuming that MX is completely dissociated in solution, what is the value of its Ksp?

Verified Solution
Key Concepts
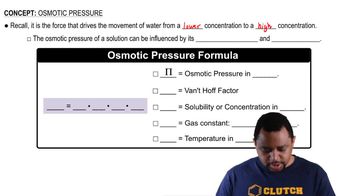
Osmotic Pressure
Dissociation of Ionic Compounds

Solubility Product Constant (Ksp)

A 40.0 mL sample of a mixture of HCl and H3PO4 was titrated with 0.100 M NaOH. The first equivalence point was reached after 88.0 mL of base, and the second equiva-lence point was reached after 126.4 mL of base. (f) What indicators would you select to signal the equiva-lence points?
In qualitative analysis, Ca2+ and Ba2+ are seperated from Na+, K+, Mg2+ by adding aqueous (NH4)2CO3 to a solution that also contains aqueous NH3 (Figure 17.18). Assume that the concentrations after mixing are 0.080 M (NH4)2CO3 and 0.16 M NH3. (a) List all the Bronsted-Lowry acids and bases present initially, and identify the principal reaction.
A railroad tank car derails and spills 36 tons of concen-trated sulfuric acid. The acid is 98.0 mass% H2SO4 and has a density of 1.836 g/mL. (a) What is the molarity of the acid?
A railroad tank car derails and spills 36 tons of concentrated sulfuric acid. The acid is 98.0 mass% H2SO4 and has a density of 1.836 g/mL. (b) How many kilograms of sodium carbonate are needed to completely neutralize the acid?
