Treatment of 1.385 g of an unknown metal M with an excess of aqueous HCl evolved a gas that was found to have a volume of 382.6 mL at 20.0 °C and 755 mm Hg pressure. Heating the reaction mixture to evaporate the water and remaining HCl then gave a white crystalline compound, MClx. After dis- solving the compound in 25.0 g of water, the melting point of the resulting solution was - 3.53 °C. (e) What are the formula and molecular weight of MClx?
A solution prepared by dissolving 100.0 g of a mixture of sugar 1C12H22O112 and table salt (NaCl) in 500.0 g of water has a freezing point of - 2.25 °C. What is the mass of each individual solute? Assume that NaCl is completely dissociated.

Verified Solution
Key Concepts
Freezing Point Depression

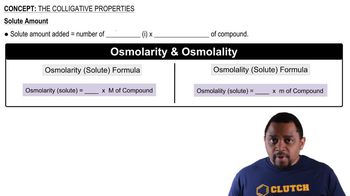
Colligative Properties
Dissociation of Ionic Compounds

Treatment of 1.385 g of an unknown metal M with an excess of aqueous HCl evolved a gas that was found to have a volume of 382.6 mL at 20.0 °C and 755 mm Hg pressure. Heating the reaction mixture to evaporate the water and remaining HCl then gave a white crystalline compound, MClx. After dis- solving the compound in 25.0 g of water, the melting point of the resulting solution was - 3.53 °C. (f) What is the identity of the metal M?
A compound that contains only C and H was burned in excess O2 to give CO2 and H2O. When 0.270 g of the com- pound was burned, the amount of CO2 formed reacted completely with 20.0 mL of 2.00 M NaOH solution according to the equation 2 OH-1aq2 + CO21g2 S CO 2- 1aq2 + H2O1l2 When 0.270 g of the compound was dissolved in 50.0 g of camphor, the resulting solution had a freezing point of 177.9 °C. [#Pure camphor freezes at 179.8 °C and has Kf = 37.7 1°C kg2>mol.] (a) What is the empirical formula of the compound?
