Here are the essential concepts you must grasp in order to answer the question correctly.
Molality vs. Molarity
Molality (m) is defined as the number of moles of solute per kilogram of solvent, while molarity (M) is the number of moles of solute per liter of solution. Molality is temperature-independent, making it more suitable for colligative properties like boiling-point elevation and freezing-point depression, which depend on the mass of the solvent rather than the volume of the solution.
Recommended video:
Colligative Properties
Colligative properties are physical properties of solutions that depend on the number of solute particles in a given amount of solvent, not the identity of the solute. Boiling-point elevation and freezing-point depression are examples of colligative properties, where the addition of solute alters the solvent's boiling and freezing points based on the concentration of solute particles.
Recommended video:
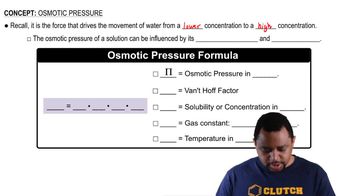
Osmotic Pressure
Osmotic pressure is the pressure required to prevent the flow of solvent into a solution through a semipermeable membrane. It is directly proportional to the molarity of the solute in the solution, making molarity a suitable measure for osmotic pressure calculations. This relationship arises because osmotic pressure depends on the concentration of solute particles in the solution, which is effectively represented by molarity.
Recommended video: