A cube-shaped crystal of an alkali metal, 1.62 mm on an edge, was vaporized in a 500.0 mL evacuated flask. The resulting vapor pressure was 12.5 mm Hg at 802 °C. The structure of the solid metal is known to be body-centered cubic. (b) Use the data in Figure 5.19 to identify the alkali metal.
Europium(II) oxide is a semiconductor with a band gap of 108 kJ/mol. Below 69 K, it is also ferromagnetic, meaning all the unpaired electrons on europium are aligned in the same direction. How many f electrons are present on each europium ion in EuO? (In lanthanide ions the 4f orbitals are lower in energy than the 6s orbitals.)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Electron Configuration

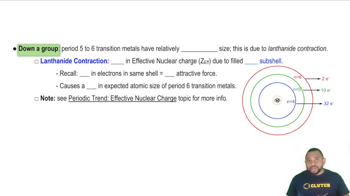
Lanthanide Contraction
Ferromagnetism
A cube-shaped crystal of an alkali metal, 1.62 mm on an edge, was vaporized in a 500.0 mL evacuated flask. The resulting vapor pressure was 12.5 mm Hg at 802 °C. The structure of the solid metal is known to be body-centered cubic. (c) What are the densities of the solid and the vapor in g>cm3?
The mineral wustite is a nonstoichiometric iron oxide with the empirical formula FexO, where x is a number slightly less than 1. Wustite can be regarded as an FeO in which some of the Fe sites are vacant. It has a density of 5.75 g>cm3, a cubic unit cell with an edge length of 431 pm, and a facecentered cubic arrangement of oxygen atoms. (c) Each Fe atom in wustite is in either the +2 or the +3 oxidation state. What percent of the Fe atoms are in the +3 oxidation state?
The mineral wustite is a nonstoichiometric iron oxide with the empirical formula FexO, where x is a number slightly less than 1. Wustite can be regarded as an FeO in which some of the Fe sites are vacant. It has a density of 5.75 g>cm3, a cubic unit cell with an edge length of 431 pm, and a facecentered cubic arrangement of oxygen atoms. (d) Using X rays with a wavelength of 70.93 pm, at what angle would third-order diffraction be observed from the planes of atoms that coincide with the faces of the unit cells? Third-order diffraction means that the value of n in the Bragg equation is equal to 3.
