Assume that you have a cylinder with a movable piston. What would happen to the gas pressure inside the cylinder if you were to do the following? (c) Decrease the volume by 45% at constant T
Ch.10 - Gases: Their Properties & Behavior
Chapter 10, Problem 49b
Assume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (b) Increase the amount of gas by one-fourth while holding the temperature and pressure constant

Verified Solution
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and amount of gas in a system through the equation PV = nRT. In this context, 'n' represents the number of moles of gas, 'R' is the ideal gas constant, and 'T' is the temperature in Kelvin. Understanding this law is crucial for predicting how changes in the amount of gas affect its volume when temperature and pressure are held constant.
Recommended video:
Guided course

Ideal Gas Law Formula
Avogadro's Principle
Avogadro's Principle states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules. This principle implies that if the amount of gas in a cylinder is increased by one-fourth while keeping temperature and pressure constant, the volume of the gas must also increase proportionally to accommodate the additional gas molecules.
Recommended video:
Guided course

Uncertainty Principle Formula
Charles's Law
Charles's Law describes how the volume of a gas is directly proportional to its temperature when pressure is held constant. Although this scenario keeps temperature constant, understanding this law helps clarify the relationship between volume and the amount of gas. It reinforces the idea that changes in the quantity of gas will affect its volume under constant conditions.
Recommended video:
Guided course
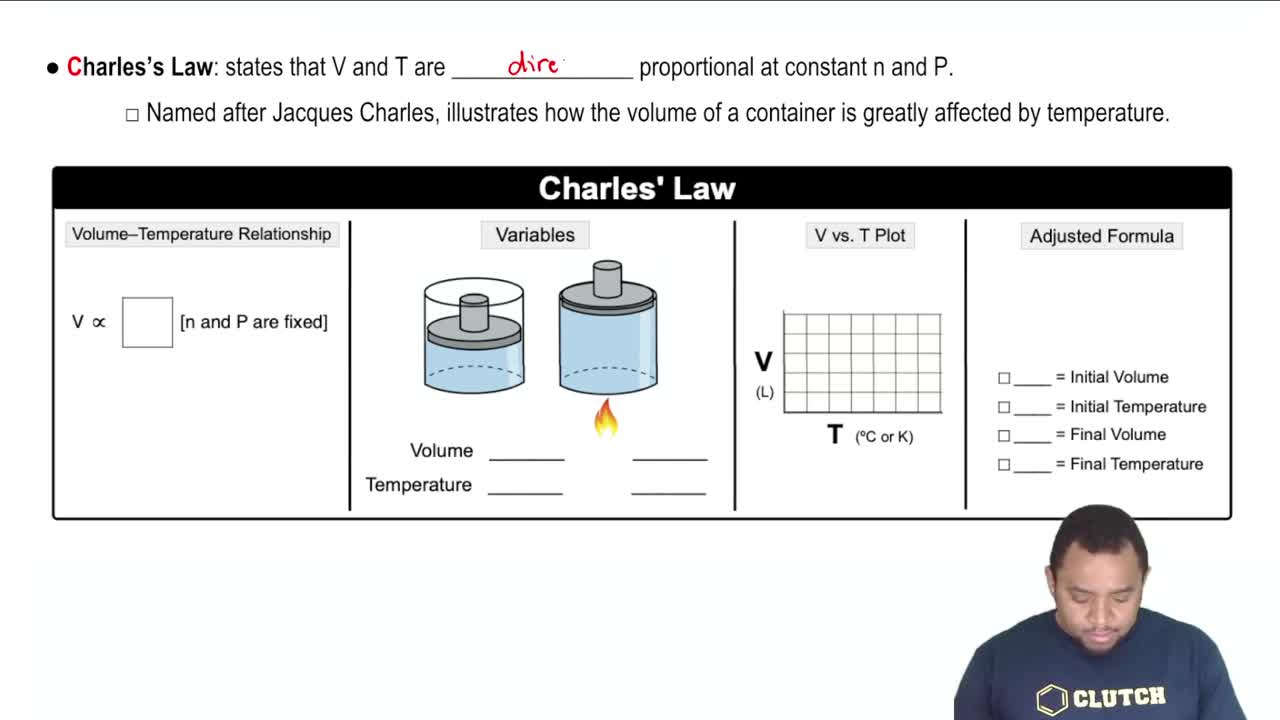
Charles's Law
Related Practice
Textbook Question
682
views
Textbook Question
Assume that you have a cylinder with a movable piston. What would happen to the gas pressure inside the cylinder if you were to do the following? (d) Halve the Kelvin temperature and triple the volume
466
views
Textbook Question
Assume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (a) Halve the Kelvin temperature while holding the pressure constant
464
views
Textbook Question
Assume that you have a cylinder with a movable piston. What would happen to the gas volume of the cylinder if you were to do the following? (d) Double the Kelvin temperature and double the pressure
967
views
Textbook Question
Which sample contains more molecules: 1.00 L of O2 at STP,
1.00 L of air at STP, or 1.00 L of H2 at STP?
983
views
Textbook Question
A compressed air tank carried by scuba divers has a volume
of 8.0 L and a pressure of 140 atm at 20 °C. What is the
volume of air in the tank in liters at STP?
1679
views
