Here are the essential concepts you must grasp in order to answer the question correctly.
Internal Energy (ΔE)
Internal energy (ΔE) is the total energy contained within a system, encompassing kinetic and potential energy at the molecular level. It reflects the energy changes that occur during chemical reactions or physical processes. Understanding ΔE is crucial for analyzing energy transfers and transformations in thermodynamics.
Recommended video:
Enthalpy (ΔH)
Enthalpy (ΔH) is a thermodynamic quantity that represents the total heat content of a system at constant pressure. It accounts for internal energy and the work done by the system due to volume changes. ΔH is particularly important in chemical reactions, as it indicates whether a reaction is exothermic or endothermic.
Recommended video:
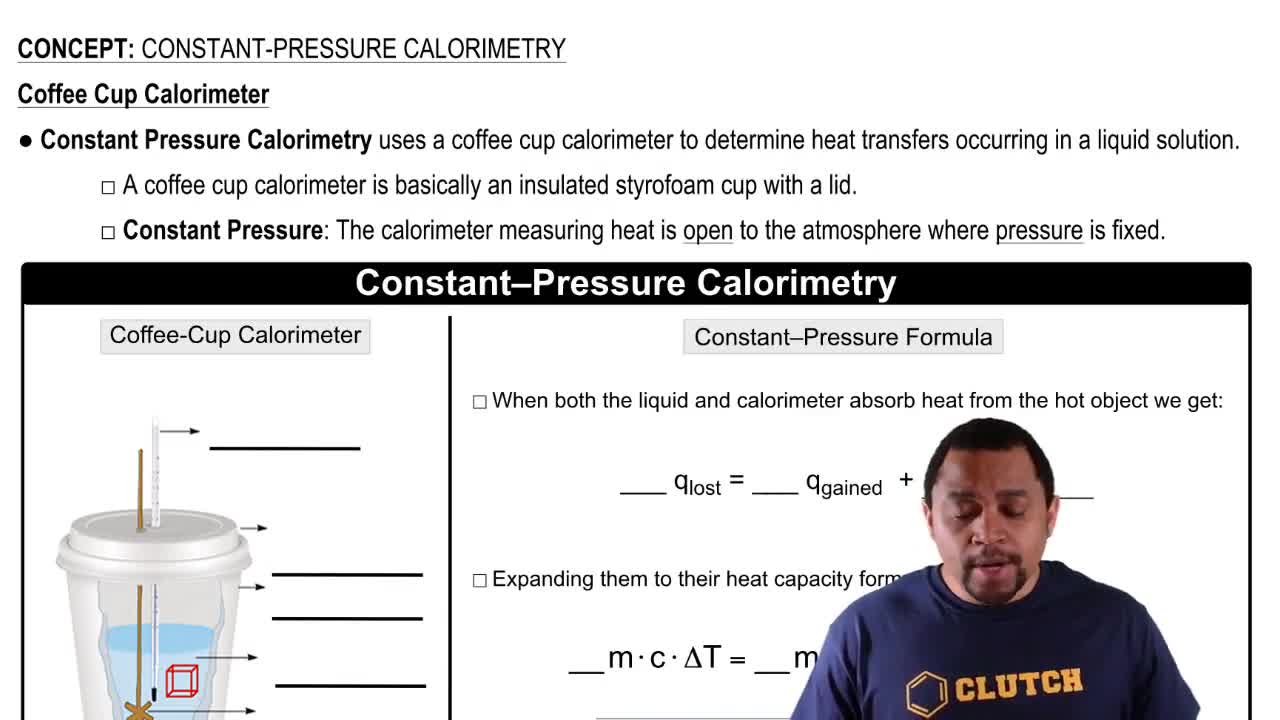
Constant Pressure Conditions
Under constant pressure conditions, the change in enthalpy (ΔH) is equal to the heat exchanged (q) during a process. When a reaction occurs in an open system at atmospheric pressure, the internal energy change (ΔE) and enthalpy change (ΔH) can be considered equal, especially when the volume change is negligible, allowing for simplified calculations in thermodynamics.
Recommended video:
Constant-Pressure Calorimetry