Here are the essential concepts you must grasp in order to answer the question correctly.
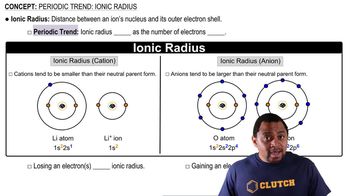
Ionic Radius
Ionic radius refers to the size of an ion in a crystal lattice. Cations (positively charged ions) are generally smaller than their neutral atoms due to the loss of electrons, which reduces electron-electron repulsion and allows the remaining electrons to be pulled closer to the nucleus. Conversely, anions (negatively charged ions) are larger than their neutral atoms because the addition of electrons increases repulsion among them, causing the electron cloud to expand.
Recommended video:
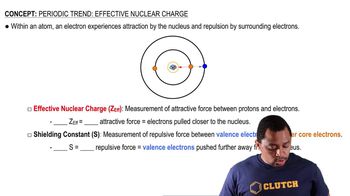
Effective Nuclear Charge (Z_eff)
Effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. It accounts for the shielding effect of inner-shell electrons that reduce the full nuclear charge felt by outer-shell electrons. A higher Z_eff leads to a stronger attraction between the nucleus and the electrons, resulting in a smaller ionic radius for cations, while a lower Z_eff in anions leads to a larger ionic radius.
Recommended video:
Isoelectronic Series
An isoelectronic series consists of ions or atoms that have the same number of electrons but different nuclear charges. In the case of Ti4+, Sc3+, Ca2+, and S2-, all have 18 electrons, but their nuclear charges differ. The size of the ions in this series decreases with increasing nuclear charge, as the greater positive charge pulls the electron cloud closer to the nucleus, resulting in smaller ionic radii for cations compared to anions.
Recommended video:
 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 54
Problem 54 Verified step by step guidance
Verified step by step guidance