Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Structure
Atoms consist of protons, neutrons, and electrons. Protons are positively charged particles found in the nucleus, while electrons are negatively charged and orbit the nucleus. The number of protons defines the element, and in a neutral atom, the number of electrons equals the number of protons.
Recommended video:
Ions
Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a net charge. Cations are positively charged ions formed by losing electrons, while anions are negatively charged ions formed by gaining electrons. The charge of an ion indicates the difference between the number of protons and electrons.
Recommended video:
Charge Calculation
To determine the number of protons and electrons in an ion, one must consider its charge. For example, a Be2+ ion has lost two electrons compared to its neutral state. Since beryllium (Be) has 4 protons, a Be2+ ion will have 4 protons and 2 electrons, reflecting its positive charge.
Recommended video:
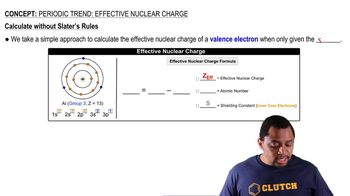
Effective Nuclear Charge Calculation