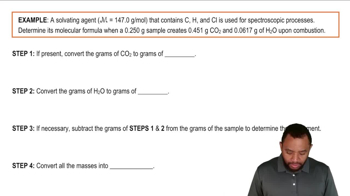
Here are the essential concepts you must grasp in order to answer the question correctly.
Spectroscopy
Spectroscopy is the study of how matter interacts with electromagnetic radiation. It involves measuring the absorption, emission, or scattering of light by substances to identify their composition and concentration. Different types of spectroscopy, such as UV-Vis, IR, and NMR, utilize specific regions of the electromagnetic spectrum to provide insights into molecular structure and behavior.
Recommended video:
Molecular Formula via Combustion Example
Continuous vs. Line Emission Spectrum
A continuous emission spectrum displays a seamless range of wavelengths, indicating that all colors of light are emitted. In contrast, a line emission spectrum consists of distinct lines at specific wavelengths, representing the emission of light from atoms or molecules transitioning between energy levels. The type of spectrum observed can provide information about the electronic structure of the substance being analyzed.
Recommended video:
Absorbance Spectrum
An absorbance spectrum is a graphical representation of how much light a substance absorbs at different wavelengths. Peaks in the absorbance spectrum indicate wavelengths where the substance absorbs light strongly, which corresponds to electronic transitions. Analyzing the absorbance spectrum helps in identifying the presence of specific compounds and understanding their concentration in a sample.
Recommended video: