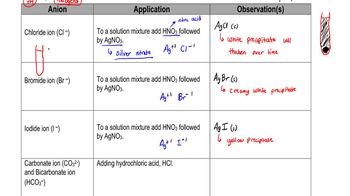
How could you use a precipitation reaction to separate each of the following pairs of anions? Write the formula for each reactant you would add, and write a balanced net ionic equation for each reaction. (a)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Precipitation Reactions

Net Ionic Equations

Anion Separation Techniques
How could you use a precipitation reaction to separate each of the following pairs of cations? Write the formula for each reactant you would add, and write a balanced net ionic equation for each reaction. (a)
How could you use a precipitation reaction to separate each of the following pairs of cations? Write the formula for each reactant you would add, and write a balanced net ionic equation for each reaction. (b)
The following three solutions are mixed: 100.0 mL of 0.100 M Na2SO4, 50.0 mL of 0.300 M ZnCl2, and 100.0 mL of 0.200 M Ba(CN)2. (a) What ionic compounds will precipitate out of solution?
The following three solutions are mixed: 100.0 mL of 0.100 M Na2SO4, 50.0 mL of 0.300 M ZnCl2, and 100.0 mL of 0.200 M Ba(CN)2. (b) What is the molarity of each ion remaining in the solution assuming complete precipitation of all insoluble compounds?
A 250.0 g sample of a white solid is known to be a mixture of KNO3, BaCl2, and NaCl. When 100.0 g of this mixture is dis-solved in water and allowed to react with excess H2SO4, 67.3 g of a white precipitate is collected. When the remaining 150.0 g of the mixture is dissolved in water and allowed to react with excess AgNO3, 197.6 g of a second precipitate is collected. (a) What are the formulas of the two precipitates?
