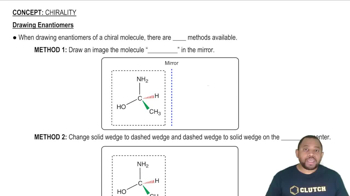
Here are the essential concepts you must grasp in order to answer the question correctly.
Isomerism
Isomerism refers to the phenomenon where two or more compounds have the same molecular formula but different structural arrangements or spatial orientations. These variations can lead to different physical and chemical properties. Isomers can be classified into structural isomers, which differ in the connectivity of atoms, and stereoisomers, which have the same connectivity but differ in the arrangement of atoms in space.
Recommended video:
Isomerism in Coordination Complexes Example
Condensed Structures
Condensed structures are a simplified way of representing molecular structures where atoms are grouped together to show connectivity without depicting all bonds explicitly. In these representations, atoms are often written in a linear format, and hydrogen atoms attached to carbons are usually omitted for clarity. This method allows for a quick understanding of the molecular framework while maintaining essential information about the compound.
Recommended video:
Line Drawings
Line drawings, also known as skeletal structures, are a graphical representation of organic molecules where carbon atoms are represented by the ends and intersections of lines, while other atoms, such as hydrogen, are often omitted for simplicity. Each vertex represents a carbon atom, and the lines represent bonds between them. This method is widely used in organic chemistry for its efficiency in conveying complex structures in a clear and concise manner.
Recommended video:
 Verified step by step guidance
Verified step by step guidance