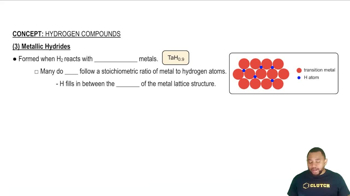
In the following pictures of binary hydrides, ivory spheres
represent H atoms or ions, and burgundy spheres represent
atoms or ions of the other element.
(1)
(2)
(3)
(4)
(a) Identify each binary hydride as ionic, covalent, or interstitial.
(b) What is the oxidation state of hydrogen in compounds (1), (2), and (3)? What is the oxidation state of the other
element?