Here are the essential concepts you must grasp in order to answer the question correctly.
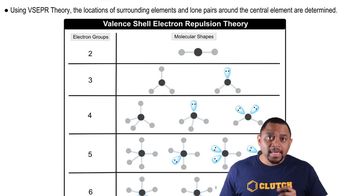
VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) Theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to VSEPR, electron pairs will arrange themselves as far apart as possible to minimize repulsion, leading to specific molecular shapes.
Recommended video:
Molecular Shapes and VSEPR
Tetrahedral Geometry
Tetrahedral geometry occurs when a central atom is bonded to four other atoms, with bond angles of approximately 109.5 degrees. In the case of SiH4, silicon is the central atom surrounded by four hydrogen atoms, resulting in a symmetrical tetrahedral shape that maximizes the distance between the bonding pairs of electrons.
Recommended video:
Hybridization
Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals that can accommodate bonding. In SiH4, the silicon atom undergoes sp3 hybridization, where one s orbital and three p orbitals combine to create four equivalent sp3 hybrid orbitals, each forming a sigma bond with a hydrogen atom.
Recommended video:
 Verified step by step guidance
Verified step by step guidance