Here are the essential concepts you must grasp in order to answer the question correctly.
Lanthanide Series
The lanthanide series consists of 15 elements from lanthanum (La) to lutetium (Lu) in the periodic table. These elements are known for their similar properties and are often referred to as rare earth elements. They are characterized by the filling of the 4f subshell, which plays a crucial role in their chemical behavior and physical properties.
Recommended video:
Lanthanide Contraction
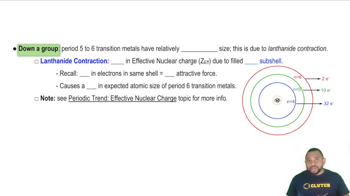
Lanthanide contraction refers to the gradual decrease in the ionic and atomic radii of the lanthanide elements as the atomic number increases. This phenomenon occurs due to the poor shielding effect of the 4f electrons, which leads to a stronger effective nuclear charge felt by the outer electrons. As a result, the elements become more compact despite the addition of more electrons.
Recommended video:
Effective Nuclear Charge
Effective nuclear charge (Z_eff) is the net positive charge experienced by an electron in a multi-electron atom. It accounts for the actual nuclear charge minus the shielding effect of inner-shell electrons. In the context of the lanthanides, the increase in Z_eff as one moves across the series contributes to the lanthanide contraction, as the outer electrons are pulled closer to the nucleus, reducing atomic size.
Recommended video:
 Verified step by step guidance
Verified step by step guidance