Six isomers for a square planar palladium(II) complex that contains two Cl-and two SCN-ligands are shown below.
(a) Which structures are cis-trans isomers?
(b) Which structures are linkage isomers?
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.87b
Problem 21.87b Verified step by step guidance
Verified step by step guidance

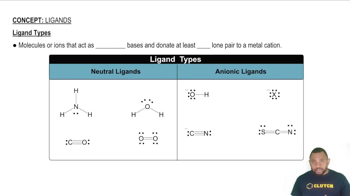
Six isomers for a square planar palladium(II) complex that contains two Cl-and two SCN-ligands are shown below.
(a) Which structures are cis-trans isomers?
(b) Which structures are linkage isomers?
Which of the following complexes can exist as diastereoisomers?
(a) [Cr(NH3)2Cl4]-
(b) [Co(NH3)5Br]2+
(c) [MnCl2Br2]2- (tetrahedral)
(d) [Pt(NH3)2Br2]2-
Tell how many diastereoisomers are possible for each of the following complexes, and draw their structures.
(a) Pt(NH3)3Cl (square planar)
(b) [FeBr2Cl2(en)]-
Which of the following complexes can exist as enantiomers? Draw their structures.
(a) [Cr(en)3]3+
(b) cis-[Co(NH3)Cl]2+
(c) trans-[Co(en)2(NH3)Cl]2+
(d) [Pt(NH3)3Cl3]+
What is the crystal field energy level diagram for the complex [Fe(NH3)6]3+?
(a)
(b)
(c)
(d)
Draw all possible diastereoisomers of [Cr(C2O4)2(H2O)2]-. Which can exist as a pair of enantiomers?