Which of the following complexes are chiral?
(a) Pt(en)Cl2
(b) cis-[Co(NH3)4Br2]+
(c) cis-[Cr(en)2(H2O)2]3+
(d) [Cr(C2O4)3]3-
 Verified step by step guidance
Verified step by step guidance


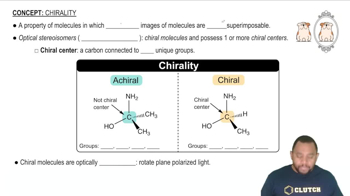
Which of the following complexes are chiral?
(a) Pt(en)Cl2
(b) cis-[Co(NH3)4Br2]+
(c) cis-[Cr(en)2(H2O)2]3+
(d) [Cr(C2O4)3]3-
Tris(2-aminoethyl)amine, abbreviated tren, is the tetradentate ligand N(CH2CH2NH2)3. Using to represent each of the three NCH2CH2NH2 segments of the ligand, sketch all possible isomers of the octahedral complex [Co(tren)BrCl]+.
The reaction of the octahedral complex Co(NH3)3(NO2)3 with HCl yields a complex [Co(NH3)3(H2O)Cl2]+ in which the two chloride ligands are trans to one another.
(a) Draw the two possible stereoisomers of the starting material [Co(NH3)3(NO2)3]. (All three NO2- ligands are bonded to Co through the N atom.)
(b) Assuming that the NH3 groups remain in place, which of the two starting isomers could give rise to the observed product?