Here are the essential concepts you must grasp in order to answer the question correctly.
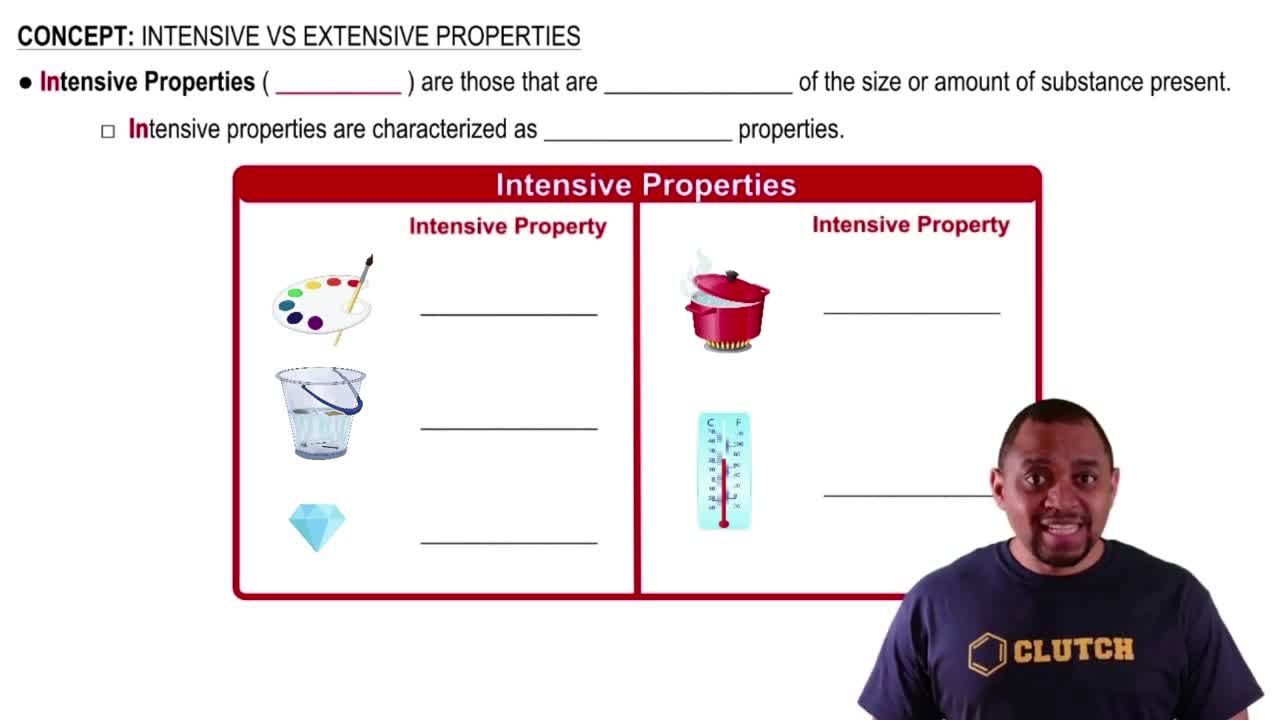
Intensive Properties
Intensive properties are characteristics of a substance that do not change regardless of the amount of the substance present. Examples include temperature, density, and boiling point. These properties are intrinsic to the material itself and are useful for identifying substances.
Recommended video:
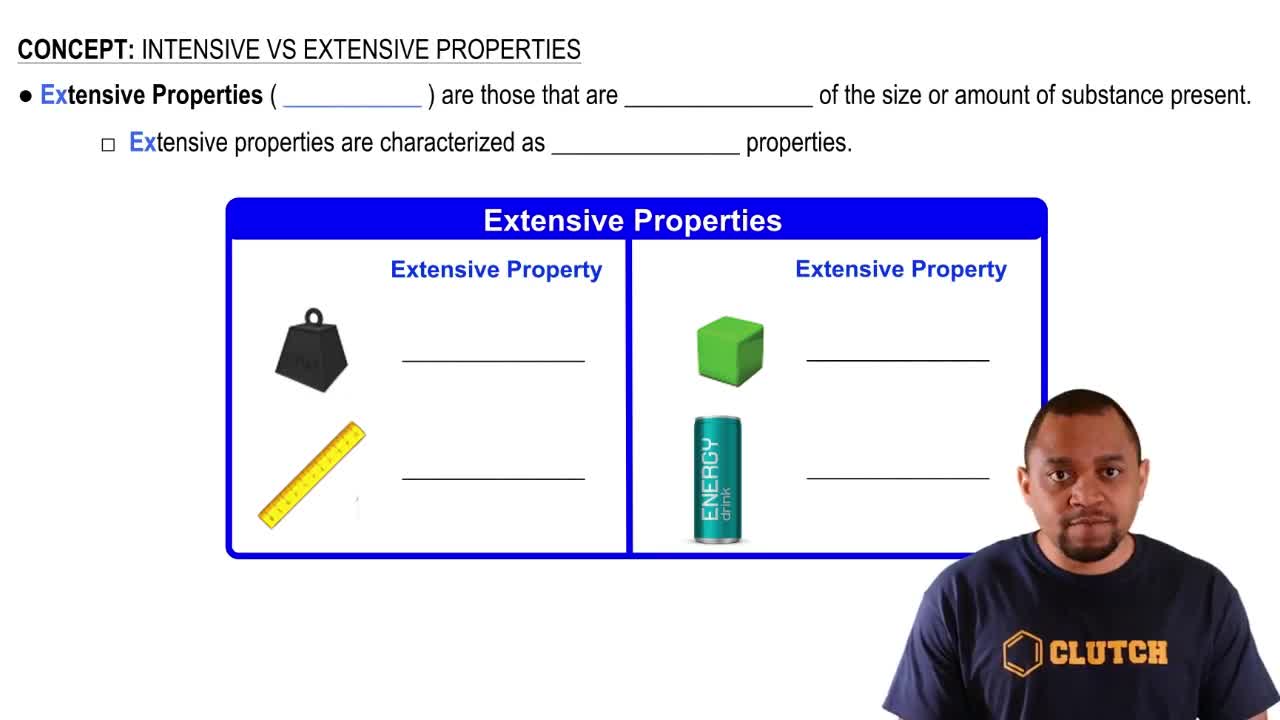
Extensive Properties
Extensive properties are characteristics that do depend on the amount of substance present. Examples include mass, volume, and total energy. These properties change when the size or quantity of the material changes, making them less useful for identifying substances.
Recommended video:
Difference Between Intensive and Extensive Properties
The key difference between intensive and extensive properties lies in their dependence on the quantity of material. Intensive properties remain constant regardless of the amount, while extensive properties vary with the size of the sample. Understanding this distinction is crucial for classifying and analyzing substances in chemistry.
Recommended video:
 Verified step by step guidance
Verified step by step guidance