Here are the essential concepts you must grasp in order to answer the question correctly.
Periodic Law
The Periodic Law states that the properties of elements are a periodic function of their atomic numbers. Mendeleev organized his periodic table based on this principle, arranging elements in order of increasing atomic mass while grouping them by similar chemical properties. This organization allowed for the prediction of undiscovered elements and their properties.
Recommended video:
Atomic Mass
Atomic mass is the weighted average mass of an element's isotopes, measured in atomic mass units (amu). Mendeleev initially used atomic mass as the primary criterion for arranging elements in his periodic table. This approach highlighted the relationship between an element's mass and its chemical behavior, although modern periodic tables are organized by atomic number.
Recommended video:
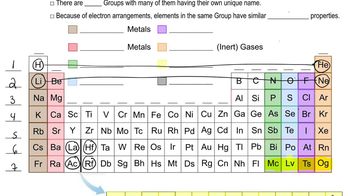
Group and Period
In the context of the periodic table, a group refers to a vertical column of elements that share similar chemical properties, while a period refers to a horizontal row. Mendeleev's table grouped elements with analogous characteristics, allowing for easier identification of trends and relationships among elements. This structural organization is fundamental to understanding the behavior of elements in chemical reactions.
Recommended video:
Periodic Table: Group Names
 Verified step by step guidance
Verified step by step guidance