Here are the essential concepts you must grasp in order to answer the question correctly.
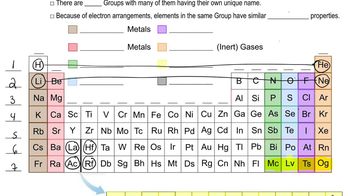
Alkaline Earth Metals
Alkaline earth metals are a group of elements found in Group 2 of the periodic table. They are characterized by having two electrons in their outermost shell, which makes them highly reactive, though less so than alkali metals. The five common alkaline earth metals include beryllium, magnesium, calcium, strontium, and barium. These metals typically form +2 cations and are known for their metallic properties.
Recommended video:
Periodic Table Groups
The periodic table is organized into columns called groups, which contain elements with similar chemical properties. Group 2, known as the alkaline earth metals, includes elements that share common characteristics such as reactivity and the formation of basic oxides. Understanding the organization of the periodic table helps in predicting the behavior of elements and their compounds based on their group affiliations.
Recommended video:
Periodic Table: Group Names
Reactivity of Metals
The reactivity of metals refers to how readily they undergo chemical reactions, often by losing electrons to form positive ions. Alkaline earth metals are more reactive than transition metals but less reactive than alkali metals. Their reactivity increases down the group, meaning that heavier alkaline earth metals like barium are more reactive than lighter ones like beryllium. This property is crucial for understanding their applications and behavior in chemical reactions.
Recommended video:
 Verified step by step guidance
Verified step by step guidance