Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactive Decay
Radioactive decay is the process by which unstable atomic nuclei lose energy by emitting radiation. This can occur in various forms, including alpha (α) and beta (β) decay. In alpha decay, the nucleus emits an alpha particle, which consists of two protons and two neutrons, while beta decay involves the transformation of a neutron into a proton with the emission of a beta particle (an electron or positron).
Recommended video:
Rate of Radioactive Decay
Alpha and Beta Particles
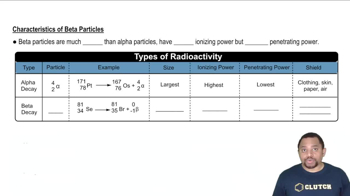
Alpha particles are positively charged and consist of two protons and two neutrons, effectively reducing the atomic number of the parent nucleus by two and the mass number by four. Beta particles, on the other hand, are high-energy, high-speed electrons or positrons emitted from a decaying nucleus, which increases the atomic number by one while keeping the mass number unchanged. Understanding these emissions is crucial for determining the final stable nucleus after decay.
Recommended video:
Characteristics of Beta Particles
Decay Series and Stability
A decay series is a sequence of radioactive decays that a particular isotope undergoes until it reaches a stable isotope. Each decay step alters the atomic and mass numbers, leading to different isotopes. The stability of the final nucleus is determined by its position on the nuclear stability curve, where isotopes with a balanced ratio of protons to neutrons are more stable. In the case of uranium-238, the series ultimately leads to a stable isotope of lead.
Recommended video:
Band of Stability: Beta Decay
 Verified step by step guidance
Verified step by step guidance