Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Fission
Nuclear fission is the process by which a heavy nucleus, such as uranium-235, splits into two smaller nuclei along with the release of energy and neutrons. This reaction is fundamental to both nuclear power plants and nuclear weapons, but the conditions and materials used differ significantly. In power plants, controlled fission generates energy for electricity, while in weapons, an uncontrolled chain reaction leads to an explosive release of energy.
Recommended video:
Band of Stability: Nuclear Fission
Uranium Enrichment
Uranium enrichment is the process of increasing the percentage of the isotope uranium-235 in uranium ore. For nuclear power plants, uranium is typically enriched to about 3-5% U-235, which is sufficient for sustaining a controlled fission reaction. In contrast, nuclear weapons require uranium enriched to about 90% U-235 to achieve the rapid, uncontrolled fission necessary for an explosion.
Recommended video:
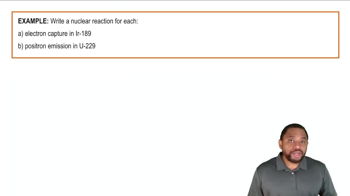
Electron Capture & Positron Emission Reaction Example
Critical Mass
Critical mass is the minimum amount of fissile material needed to maintain a nuclear chain reaction. In the context of nuclear weapons, achieving critical mass involves bringing together enough fissionable material, such as uranium-235, to initiate an explosive reaction. This concept is crucial for understanding how nuclear weapons function, as it determines the design and efficiency of the weapon.
Recommended video: