In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (b) How many coulombs of electrical charge are needed?
Ch.19 - Electrochemistry
Chapter 19, Problem 148
Consider the following half-reactions and E° values: (c) Write the cell reaction for part (b), and calculate the values of E°, ∆G° (in kilojoules), and K for this reaction at 25 °C
 Verified step by step guidance
Verified step by step guidance1
Identify the two half-reactions involved in the electrochemical cell and their respective standard reduction potentials (E°).
Write the balanced overall cell reaction by combining the two half-reactions, ensuring that the number of electrons lost in the oxidation half-reaction equals the number of electrons gained in the reduction half-reaction.
Calculate the standard cell potential (E°_cell) by subtracting the standard reduction potential of the anode (oxidation) from the standard reduction potential of the cathode (reduction): E°_cell = E°_cathode - E°_anode.
Use the Nernst equation to calculate the standard Gibbs free energy change (∆G°) for the reaction: ∆G° = -nFE°_cell, where n is the number of moles of electrons transferred and F is the Faraday constant (approximately 96485 C/mol).
Calculate the equilibrium constant (K) for the reaction at 25 °C using the relationship between ∆G° and K: ∆G° = -RTlnK, where R is the universal gas constant (8.314 J/mol·K) and T is the temperature in Kelvin (298 K).

Verified Solution
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrode Potential (E°)
Electrode potential, denoted as E°, is a measure of the tendency of a chemical species to be reduced, expressed in volts. It is determined under standard conditions (1 M concentration, 1 atm pressure, and 25 °C). The more positive the E° value, the greater the species' ability to gain electrons and undergo reduction, which is crucial for predicting the direction of electron flow in electrochemical cells.
Recommended video:
Guided course
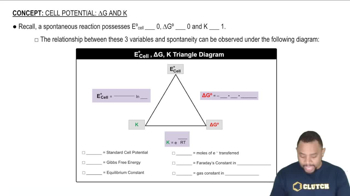
Relationship between ∆E°, ∆G°, and K
Gibbs Free Energy (∆G°)
Gibbs free energy change (∆G°) is a thermodynamic quantity that indicates the spontaneity of a reaction at standard conditions. It is calculated using the equation ∆G° = -nFE°, where n is the number of moles of electrons transferred, F is Faraday's constant, and E° is the cell potential. A negative ∆G° indicates a spontaneous reaction, while a positive value suggests non-spontaneity.
Recommended video:
Guided course

Gibbs Free Energy of Reactions
Equilibrium Constant (K)
The equilibrium constant (K) quantifies the ratio of the concentrations of products to reactants at equilibrium for a given reaction. It is related to the Gibbs free energy change by the equation ∆G° = -RT ln(K), where R is the universal gas constant and T is the temperature in Kelvin. A larger K value indicates a reaction that favors product formation, while a smaller K suggests reactants are favored.
Recommended video:
Guided course

Equilibrium Constant K
Related Practice
Textbook Question
191
views
Textbook Question
In order to charge a lead storage battery (Section 19.10) 500.0 g of PbSO4(s) must be converted into PbO2(s) and Pb(s). (c) If a current of 500 A is used, how long will it take?
Textbook Question
When the nickel–zinc battery, used in digital cameras, is recharged, the following cell reaction occurs: (a) How many grams of zinc are formed when 3.35 x 10-2 g of Ni(OH)2 are consumed?
281
views
Textbook Question
Chlorine can be prepared in the laboratory by the reaction of hydrochloric acid and potassium permanganate.
(b) Calculate E° and ∆G° for the reaction
602
views
Textbook Question
Chlorine can be prepared in the laboratory by the reaction of hydrochloric acid and potassium permanganate. (a) Use data in Appendix D to write a balanced equation for the reaction. The reduction product is Mn2+.
670
views
Textbook Question
The sodium–sulfur battery has molybdenum electrodes with anode and cathode compartments separated by b-alumina, a ceramic through which sodium ions can pass. Because the battery operates at temperatures above 300 °C, all the reactants and products are present in a molten solution. The cell voltage is about 2.0 V.
(b) How many kilograms of sodium are consumed when a 25 kW sodium–sulfur battery produces current for 32 min?
216
views
