Here are the essential concepts you must grasp in order to answer the question correctly.
Strong Acid vs. Weak Acid
A strong acid completely dissociates in solution, resulting in a high concentration of hydrogen ions (H+) and a low initial pH. In contrast, a weak acid only partially dissociates, leading to a higher initial pH due to the lower concentration of H+ ions. This fundamental difference affects the shape of the titration curve.
Recommended video:
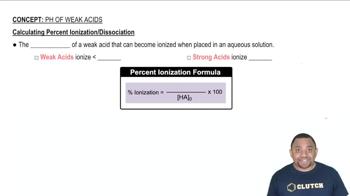
Calculating Percent Ionization of Weak Acids
Titration Curves
Titration curves graph the pH of a solution as a function of the volume of titrant added. For a strong acid-strong base titration, the curve shows a sharp increase in pH at the equivalence point, while a weak acid-strong base titration displays a more gradual slope and a buffer region before the equivalence point, reflecting the weak acid's resistance to pH changes.
Recommended video:
Acid-Base Titration Curves
Equivalence Point
The equivalence point in a titration is reached when the amount of titrant added is stoichiometrically equivalent to the amount of substance in the solution. For strong acid-strong base titrations, this point typically occurs at pH 7, while for weak acid-strong base titrations, it occurs at a pH greater than 7 due to the formation of a weak conjugate base.
Recommended video: