Here are the essential concepts you must grasp in order to answer the question correctly.
Partial Pressure
Partial pressure refers to the pressure exerted by a single component of a gas mixture. In this context, it is the pressure of oxygen in the lungs at a specific altitude, which affects how much oxygen can dissolve in blood or water. Understanding partial pressure is crucial for calculating the concentration of dissolved gases, as it directly influences solubility according to Henry's Law.
Recommended video:
Partial Pressure Calculation
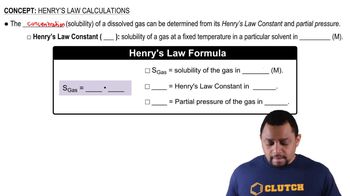
Henry's Law
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This principle is essential for determining the concentration of dissolved oxygen in blood at different altitudes and pressures. The law can be expressed mathematically as C = kH * P, where C is the concentration, kH is the Henry's Law constant, and P is the partial pressure.
Recommended video:
Solubility of Gases
The solubility of gases in liquids varies with temperature and pressure. At 37 °C and 1 atm, the solubility of oxygen is given as 1.93 * 10^-3 mol/L. This value indicates how much oxygen can dissolve in water under standard conditions, and it is essential for calculating the concentration of dissolved oxygen at different partial pressures, such as the one at 10,000 ft.
Recommended video: