Here are the essential concepts you must grasp in order to answer the question correctly.
Amorphous vs. Crystalline Solids
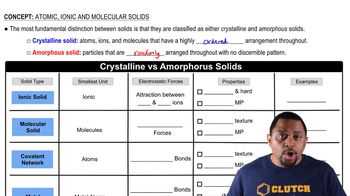
Amorphous solids lack a long-range ordered structure, resulting in a non-uniform arrangement of particles. In contrast, crystalline solids have a well-defined geometric arrangement, leading to distinct melting points and physical properties. Understanding this distinction is crucial for identifying substances like rubber, which is amorphous, versus crystalline materials.
Recommended video:
Crystalline vs Amorphous Solids
Types of Solids
Solids can be categorized into different types based on their bonding and structure: ionic solids (like Na3PO4) consist of ions held together by electrostatic forces; molecular solids (like CBr4) are formed by molecules held together by van der Waals forces; covalent network solids (like quartz) feature a continuous network of covalent bonds; and metallic solids (like Au) consist of metal atoms sharing a 'sea' of electrons. Recognizing these categories is essential for classifying the given substances.
Recommended video:
Crystalline Solids Structure
Properties of Metals and Nonmetals
Metals, such as gold (Au), are characterized by their ability to conduct electricity and heat, malleability, and ductility due to the delocalized electrons in their structure. Nonmetals, including substances like quartz and rubber, exhibit different properties, such as brittleness and poor conductivity. Understanding these properties helps in identifying which substances fit the descriptions provided in the question.
Recommended video:
 Verified step by step guidance
Verified step by step guidance