Here are the essential concepts you must grasp in order to answer the question correctly.
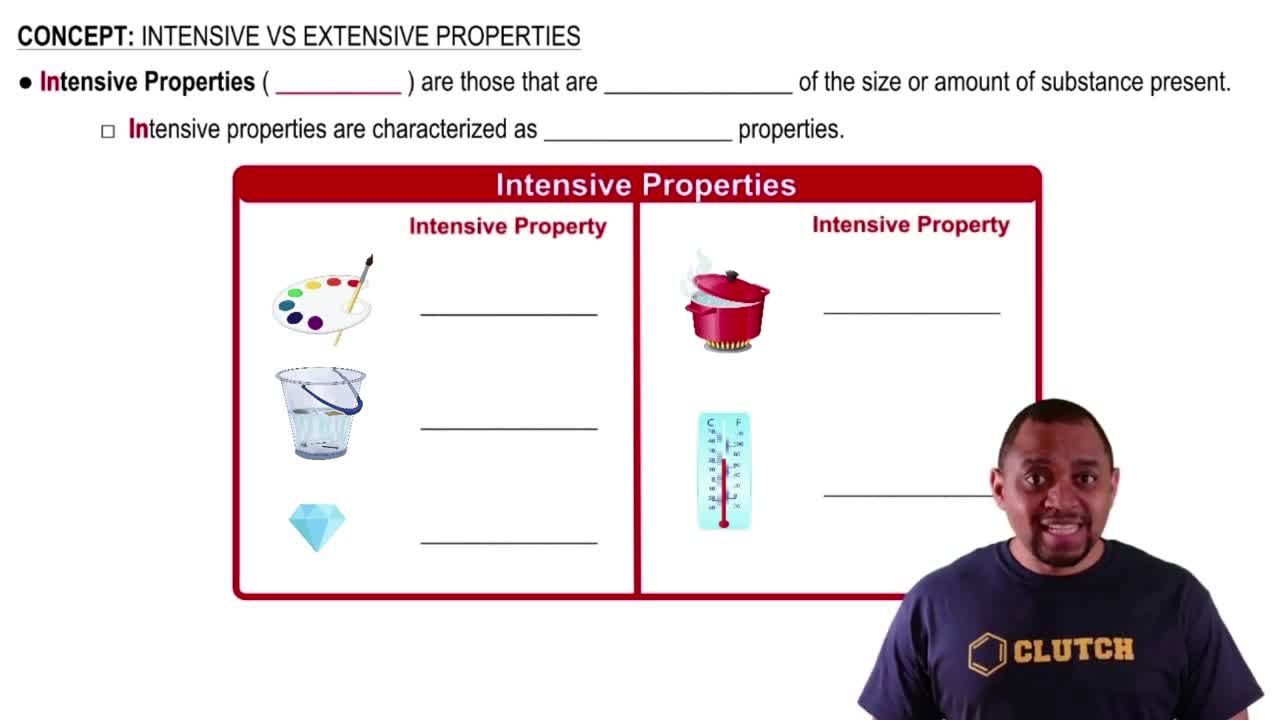
Intensive Properties
Intensive properties are characteristics of a substance that do not depend on the amount of material present. Examples include density, boiling point, and electrical conductivity. These properties remain constant regardless of the sample size, making them useful for identifying substances.
Recommended video:
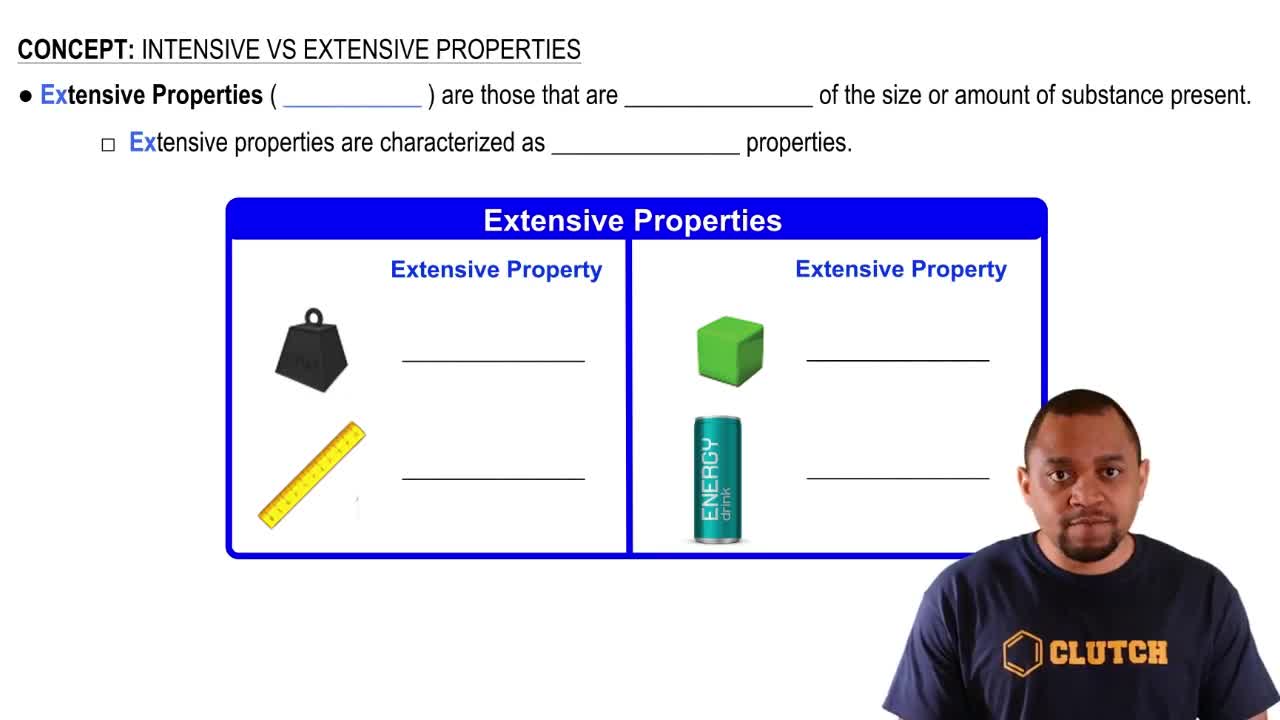
Extensive Properties
Extensive properties are characteristics that do depend on the amount of material in a sample. Common examples include mass and volume. As the quantity of the substance increases, these properties also increase, which is important for calculations involving the total amount of material.
Recommended video:
Density
Density is defined as the mass of a substance per unit volume, typically expressed in grams per cubic centimeter (g/cm³) or kilograms per liter (kg/L). It is an intensive property, meaning it remains constant regardless of the sample size. Density is crucial for distinguishing between different materials and understanding their behavior in various contexts.
Recommended video:
 Verified step by step guidance
Verified step by step guidance