The following is part of a molecular orbital energy-level diagram for MOs constructed from 1s atomic orbitals.
(a) What labels do we use for the two MOs shown?
 Verified step by step guidance
Verified step by step guidance

The following is part of a molecular orbital energy-level diagram for MOs constructed from 1s atomic orbitals.
(a) What labels do we use for the two MOs shown?
a. Methane (CH4) and the perchlorate ion (ClO4−) are both described as tetrahedral. What does this indicate about their bond angles?
b. The NH3 molecule is trigonal pyramidal, while BF3 is trigonal planar. Which of these molecules is flat?
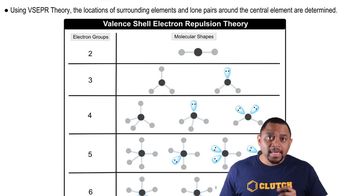
(b) An AB4 molecule has two lone pairs of electrons on the A atom (in addition to the four B atoms). What is the electron-domain geometry around the A atom?
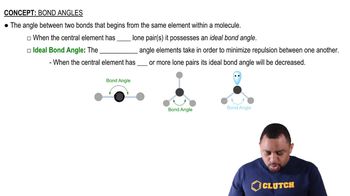
Would you expect the nonbonding electron-pair domain in NH3 to be greater or less in size than the corresponding one in PH3?
In which of the following molecules can you confidently predict the bond angles about the central atom, and for which would you be a bit uncertain? Explain in each case. (a) H2S, (b) BCl3, (c) CH3I, (d) CBr4, (e) TeBr4.