The orbital diagram that follows presents the final step in the formation of hybrid orbitals by a silicon atom. (b) What type of hybrid orbital is produced in this hybridization?
Consider the following hydrocarbon:
d. Identify all the 120° bond angles in the molecule.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
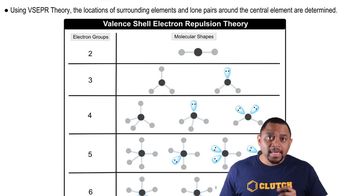
Molecular Geometry

VSEPR Theory
Hybridization

Consider the following hydrocarbon:
a. What is the hybridization at each carbon atom in the molecule?
Consider the following hydrocarbon:
b. How many 𝜎 bonds are there in the molecule?
The drawing below shows the overlap of two hybrid orbitals to form a bond in a hydrocarbon. (a) Which of the following types of bonds is being formed: (i) C¬C s, (ii) C¬C p, or (iii) C¬H s?
The drawing below shows the overlap of two hybrid orbitals to form a bond in a hydrocarbon. (b) Which of the following could be the identity of the hydrocarbon: (i) CH4, (ii) C2H6, (iii) C2H4, or (iv) C2H2?
The molecule shown here is called furan. It is represented in the typical shorthand way for organic molecules, with hydrogen atoms not shown, and each of the four vertices representing a carbon atom. e. The bond angles in furan are much smaller than those in benzene. The likely reason is which of the following? i. The hybridization of the carbon atoms in furan is different from that in benzene. ii. Furan does not have another resonance structure equivalent to the one shown here. iii. The atoms are forced to adopt smaller angles in a five-membered ring than in a six-membered ring. [Section 9.5]
