Indicate whether each statement is true or false. (d) Electrons cannot occupy a nonbonding orbital.
a. Which of the following is expected to be paramagnetic: Ne, Li2, Li2+, N2, N2+, N22−? b. For each of the substances in part (a) that is paramagnetic, determine the number of unpaired electrons it has.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Paramagnetism
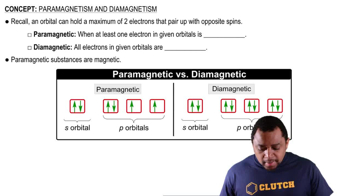
Electron Configuration

Molecular Orbital Theory

a. Based on its molecular-orbital diagram, what is the bond order of the O2 molecule?
b. What is the expected bond order for the peroxide ion, O22−?
c. What is the expected bond order for the superoxide ion, O2−?
d. From shortest to longest, predict the ordering of the bond lengths for O2, O22−, and O2−.
e. From weakest to strongest, predict the ordering of the bond strengths for O2, O22−, and O2−.
Determine whether each of the following statements about diamagnetism and paramagnetism is true or false:
a. A diamagnetic substance is weakly repelled from a magnetic field.
b. A substance with unpaired electrons will be diamagnetic.
c. A paramagnetic substance is attracted to a magnetic field.
d. The O2 molecule is paramagnetic.
Using Figures 9.39 and 9.43 as guides, draw the molecular-orbital electron configuration for (d) Ne22+. In each case indicate whether the addition of an electron to the ion would increase or decrease the bond order of the species.
If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of b. NO–
If we assume that the energy-level diagrams for homonuclear diatomic molecules shown in Figure 9.43 can be applied to heteronuclear diatomic molecules and ions, predict the bond order and magnetic behavior of d. NeF+
