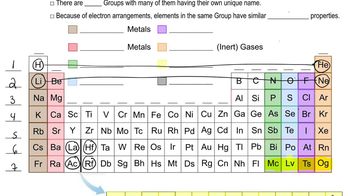
(a) True or false: An element's number of valence electrons is the same as its atomic number.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Valence Electrons

Atomic Number

Periodic Table Groups
The molecule shown here is styrene, C8H8, a benzene derivative that is used to make a number of polymers, including polystyrene. The shorthand notation for the benzene ring (described in Section 8.6) is used. Three of the carbon–carbon bonds are numbered in the structure.
a. Which of the three bonds is the strongest?
b. Which of the three bonds is the longest?
c. Which of the three bonds is best described as halfway between a single and a double bond? [Sections 8.6 and 8.8]
Consider the Lewis structure for the polyatomic oxyanion shown here, where X is an element from the third period (Na - Ar). By changing the overall charge, n, from 1- to 2- to 3- we get three different polyatomic ions. For each of these ions (b) determine the formal charge of the central atom, X;
(b) How many valence electrons does a nitrogen atom possess?
(c) An atom has the electron configuration 1s22s22p63s23p2. How many valence electrons does the atom have?
(a) True or false: The hydrogen atom is most stable when it has a full octet of electrons.
