Consider the isoelectronic ions F- and Na+. (d) For isoelectronic ions, how are effective nuclear charge and ionic radius related?
Arrange each of the following sets of atoms and ions in order of increasing size. Se2−,Te2−, Se
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Atomic Radius

Ionic Radius
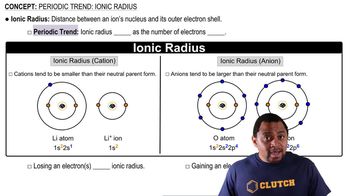
Comparison of Ions and Atoms

Consider S, Cl, and K and their most common ions. (a) List the atoms in order of increasing size.
Consider S, Cl, and K and their most common ions.(c) Explain any differences in the orders of the atomic and ionic sizes.
True or false? c. S2− is larger than K+.
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. b. Calculate the difference between the experimentally measured ion–ion distances and the ones predicted from Figure 7.8.
In the ionic compounds LiF, NaCl, KBr, and RbI, the measured cation–anion distances are 2.01 Å (Li–F), 2.82 Å (Na–Cl), 3.30 Å (K–Br), and 3.67 Å (Rb–I), respectively. c. What estimates of the cation–anion distance would you obtain for these four compounds using neutral atom bonding atomic radii? Are these estimates as accurate as the estimates using ionic radii?
