Textbook Question
(b) What are 'core electrons'?
793
views
 Verified step by step guidance
Verified step by step guidance

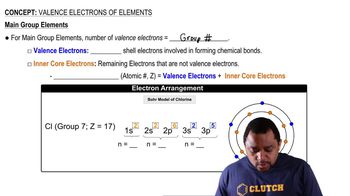

(b) What are 'core electrons'?
(c) What does each box in an orbital diagram represent?
For each element, indicate the number of valence electrons, core electrons, and unpaired electrons in the ground state: a. nitrogen
Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons each has: (a) Mg.
Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons each has: (f) Lu.
Identify the specific element that corresponds to each of the following electron configurations and indicate the number of unpaired electrons for each: (a) 1s22s2