An experiment called the Stern–Gerlach experiment helped establish the existence of electron spin. In this experiment, a beam of silver atoms is passed through a magnetic field, which deflects half of the silver atoms in one direction and half in the opposite direction. The separation between the two beams increases as the strength of the magnetic field increases. (a) What is the electron configuration for a silver atom?
Ch.6 - Electronic Structure of Atoms
Chapter 6, Problem 72
What is the maximum number of electrons in an atom that can have the following quantum numbers? a. n = 3, ml = -2; b. n = 4, l = 3; c. n = 5, l = 3, ml = 2; d. n = 4, l = 1, ml = 0.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the quantum numbers and their significance. The principal quantum number (n) indicates the energy level, the azimuthal quantum number (l) indicates the subshell, the magnetic quantum number (m_l) indicates the orientation of the orbital, and the spin quantum number (m_s) indicates the spin of the electron.
Step 2: For part (a), n = 3 and m_l = -2. Determine the possible values of l for n = 3, which are 0, 1, and 2. Since m_l = -2, l must be 2 (d subshell). Each m_l value can hold 2 electrons (one for each spin).
Step 3: For part (b), n = 4 and l = 3. The l = 3 corresponds to the f subshell. Calculate the number of orbitals using the formula 2l + 1, which gives 7 orbitals. Each orbital can hold 2 electrons.
Step 4: For part (c), n = 5, l = 3, and m_l = 2. The l = 3 corresponds to the f subshell. Since m_l = 2, it refers to one specific orbital within the f subshell. Each orbital can hold 2 electrons.
Step 5: For part (d), n = 4, l = 1, and m_l = 0. The l = 1 corresponds to the p subshell. m_l = 0 refers to one specific orbital within the p subshell. Each orbital can hold 2 electrons.

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Quantum Numbers
Quantum numbers are a set of four numbers that describe the unique quantum state of an electron in an atom. They include the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). Each quantum number provides specific information about the electron's energy level, shape of the orbital, orientation in space, and spin direction.
Recommended video:
Guided course

Principal Quantum Number
Principal Quantum Number (n)
The principal quantum number (n) indicates the main energy level or shell of an electron in an atom. It can take positive integer values (1, 2, 3, ...), with higher values corresponding to higher energy levels and greater distances from the nucleus. The maximum number of electrons that can occupy a shell is given by the formula 2n², which helps determine the electron capacity for each energy level.
Recommended video:
Guided course

Principal Quantum Number
Magnetic Quantum Number (ml)
The magnetic quantum number (ml) specifies the orientation of an orbital in space and can take integer values ranging from -l to +l, where l is the azimuthal quantum number. This means that for each value of l, there are 2l + 1 possible values of ml, which correspond to the number of orbitals within a subshell. Understanding ml is crucial for determining how many electrons can occupy a given orbital.
Recommended video:
Guided course
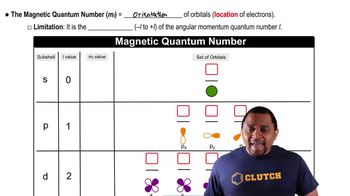
Magnetic Quantum Number
Related Practice
Textbook Question
438
views
Textbook Question
An experiment called the Stern–Gerlach experiment helped establish the existence of electron spin. In this experiment, a beam of silver atoms is passed through a magnetic field, which deflects half of the silver atoms in one direction and half in the opposite direction. The separation between the two beams increases as the strength of the magnetic field increases. (c) Would this experiment work for a beam of fluorine (F) atoms?
1490
views
Textbook Question
What is the maximum number of electrons that can occupy each of the following subshells? a. 3p, b. 5d, c. 2s, d. 4f.
2
views
Textbook Question
(a) What are 'valence electrons'?
508
views
Textbook Question
(b) What are 'core electrons'?
793
views
Textbook Question
(c) What does each box in an orbital diagram represent?
1494
views
