Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g). (b) Indicate the oxidation number for each atom involved in the reaction in part (a). What elements are being oxidized and reduced?
A coffee-cup calorimeter of the type shown in Figure 5.18 contains 150.0 g of water at 25.1°C A 121.0-g block of copper metal is heated to 100.4°C by putting it in a beaker of boiling water. The specific heat of Cu(s) is 0.385 J/g-K The Cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.1°C (d) What would be the final temperature of the system if all the heat lost by the copper block were absorbed by the water in the calorimeter?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Specific Heat Capacity

Heat Transfer

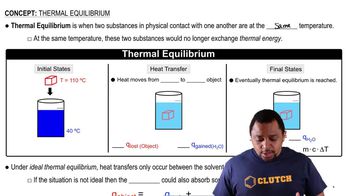
Thermal Equilibrium
A coffee-cup calorimeter of the type shown in Figure 5.18 contains 150.0 g of water at 25.1°C A 121.0-g block of copper metal is heated to 100.4°C by putting it in a beaker of boiling water. The specific heat of Cu(s) is 0.385 J/g-K The Cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.1°C. (a) Determine the amount of heat, in J, lost by the copper block.
A coffee-cup calorimeter of the type shown in Figure 5.18 contains 150.0 g of water at 25.1°C A 121.0-g block of copper metal is heated to 100.4°C by putting it in a beaker of boiling water. The specific heat of Cu(s) is 0.385 J/g-K The Cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.1°C (b) Determine the amount of heat gained by the water. The specific heat of water is 4.184 J/1gK.
(b) Assuming that there is an uncertainty of 0.002 °C in each temperature reading and that the masses of samples are measured to 0.001 g, what is the estimated uncertainty in the value calculated for the heat of combustion per mole of caffeine?
Use average bond enthalpies from Table 5.4 to estimate Δ𝐻 for the following gas-phase reaction of ethylene, (C2H4), oxygen, and hydrogen to form ethylene glycol (C2H6O2), which is the principal component of automotive antifreeze:
