Nitrogen monoxide and oxygen react to form nitrogen dioxide. Consider the mixture of NO and O2 shown in the accompanying diagram. The blue spheres represent N, and the red ones represent O. (c) If the actual yield of the reaction was 75% instead of 100%, how many molecules of each kind would be present after the reaction was over?
Ch.3 - Chemical Reactions and Reaction Stoichiometry
Chapter 3, Problem 10
A key step in balancing chemical equations is correctly identifying the formulas of the reactants and products. For example, consider the reaction between calcium oxide, CaO(s), and H2O1l2 to form aqueous calcium hydroxide. (b) Is it possible to balance the equation if you incorrectly identify the product as CaOH1aq2, and if so, what is the equation?
 Verified step by step guidance
Verified step by step guidance1
Identify the correct chemical formulas for the reactants and the incorrectly identified product: CaO(s), H_2O(l), and CaOH(aq).
Write the unbalanced chemical equation using these formulas: CaO(s) + H_2O(l) \rightarrow CaOH(aq).
Count the number of each type of atom on both sides of the equation. For the reactants, you have 1 Ca, 1 O, and 2 H atoms. For the product, you have 1 Ca, 1 O, and 1 H atom.
To balance the equation, adjust the coefficients to ensure the same number of each type of atom appears on both sides. Since the product has only 1 H atom, you need to balance the hydrogen atoms by adjusting the coefficient of H_2O.
Re-evaluate the equation after adjusting coefficients to ensure all atoms are balanced, and verify that the equation is consistent with the law of conservation of mass.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Formulas
Chemical formulas represent the composition of substances, indicating the types and numbers of atoms present. Accurate identification of reactants and products is crucial in chemical reactions, as incorrect formulas can lead to misunderstandings in the reaction process and the final balanced equation.
Recommended video:
Guided course
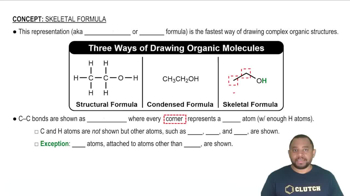
Skeletal Formula
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms for each element is the same on both sides of the equation. This reflects the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. An incorrect product formula can complicate this process, potentially leading to an unbalanced equation.
Recommended video:
Guided course

Balancing Chemical Equations
Hydroxide Ion Formation
In the context of the reaction between calcium oxide and water, the formation of hydroxide ions (OH-) is essential. The correct product, calcium hydroxide (Ca(OH)2), contains two hydroxide ions, which are crucial for the compound's properties. Misidentifying the product as CaOH could lead to confusion regarding the stoichiometry and the resulting chemical behavior.
Recommended video:
Guided course

Ion Formation
Related Practice
Textbook Question
1043
views
Textbook Question
Write 'true' or 'false' for each statement. (a) We balance chemical equations as we do because energy must be conserved.
1137
views
Textbook Question
Write 'true' or 'false' for each statement. (b) If the reaction 2 O3(g) → 3 O2(g) goes to completion and all O3 is converted to O2, then the mass of O3 at the beginning of the reaction must be the same as the mass of O2 at the end of the reaction.
434
views
Textbook Question
Balance the following equations: c. CH4(𝑔)+Cl2(𝑔)⟶CCl4(𝑙)+HCl(𝑔)
3
views
Textbook Question
Balance the following equations: b. TiCl4(𝑙)+H2O(𝑙)⟶TiO2(𝑠)+HCl(𝑎𝑞)
3
views
Textbook Question
Balance the following equations: c. NH4NO3(𝑠)⟶N2(𝑔)+O2(𝑔)+H2O(𝑔)
3
views
