Hydrazine 1N2H42 and dinitrogen tetroxide 1N2O42 form a self-igniting mixture that has been used as a rocket propellant. The reaction products are N2 and H2O. (c) Which substance serves as the reducing agent and which as the oxidizing agent?
Complete and balance the following half-reactions. In each case, indicate whether the half-reaction is an oxidation or a reduction. (f) SO32-1aq2 ¡ SO42-1aq2 (basic solution)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
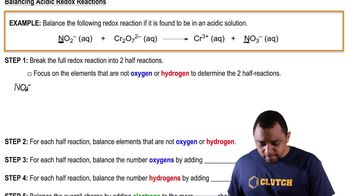
Oxidation and Reduction

Balancing Half-Reactions
Basic Solution Conditions

Complete and balance the following half-reactions. In each case, indicate whether the half-reaction is an oxidation or a reduction. (b) TiO21s2 ¡ Ti2+1aq2 (acidic solution)
Complete and balance the following half-reactions in basic solution. In each case, indicate whether the half-reaction is an oxidation or a reduction.
a. OH−(𝑎𝑞)⟶O2(𝑔)
b. SO32−(𝑎𝑞)⟶SO42−(𝑎𝑞)
c. N2(𝑔)⟶NH3(𝑔)
d. HO2−(𝑎𝑞)⟶OH−(𝑎𝑞)
Complete and balance the following half-reactions in basic solution. In each case, indicate whether the half-reaction is an oxidation or a reduction.
a. O2(𝑔)⟶H2O(𝑙)
b. Mn2+(𝑎𝑞)⟶MnO2(𝑠)
c. Cr(OH)3(𝑠)⟶CrO42−(𝑎𝑞)
d. N2H4(𝑎𝑞)⟶N2(𝑔)
Complete and balance the following half-reactions in basic solution. In each case, indicate whether the half-reaction is an oxidation or a reduction. c. Cr(OH)3(𝑠)⟶CrO42−(𝑎𝑞)
Complete and balance the following half-reactions in acidic solution. In each case indicate whether the half-reaction is an oxidation or a reduction. b. H2SO3(𝑎𝑞)⟶SO42−(𝑎𝑞)
