Write the chemical formulas for the following compounds: (g) sodium hypobromite.
Ch.2 - Atoms, Molecules, and Ions
Chapter 2, Problem 75b
Give the name or chemical formula, as appropriate, for each of the following acids: (b) HBr
 Verified step by step guidance
Verified step by step guidance1
Identify the type of compound: HBr is a binary acid, which consists of hydrogen and one other nonmetal element.
Recognize the pattern for naming binary acids: Binary acids are named using the prefix 'hydro-', followed by the root of the nonmetal element, and ending with the suffix '-ic acid'.
Determine the root of the nonmetal element: The nonmetal in HBr is bromine, so the root is 'brom'.
Apply the naming pattern: Combine the prefix 'hydro-', the root 'brom', and the suffix '-ic acid' to form the name.
Conclude with the name: The name of the acid HBr is hydrobromic acid.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acids and Their Properties
Acids are substances that can donate protons (H⁺ ions) in a solution. They typically have a sour taste and can conduct electricity when dissolved in water. The strength of an acid is determined by its ability to dissociate in water, with strong acids completely ionizing and weak acids partially ionizing.
Recommended video:
Guided course

Chemical Properties Example
Naming Acids
The naming of acids depends on the anion present in the compound. For binary acids, which consist of hydrogen and one other nonmetal, the name is formed by using the prefix 'hydro-', followed by the root of the nonmetal's name, and ending with 'ic acid'. For example, HBr is named hydrobromic acid.
Recommended video:
Guided course

Naming Binary Acids Example
Chemical Formulas
A chemical formula represents the composition of a substance, indicating the types and numbers of atoms present. For acids, the formula typically starts with hydrogen (H) followed by the anion. In the case of HBr, the formula indicates one hydrogen atom and one bromine atom, reflecting its identity as a binary acid.
Recommended video:
Guided course
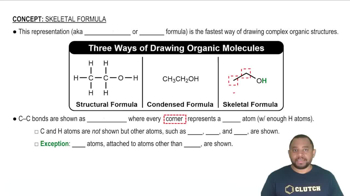
Skeletal Formula
Related Practice
Textbook Question
867
views
Textbook Question
Give the chemical formula for each of the following ionic compounds: (a) sodium phosphate (b) zinc nitrate (c) barium bromate (d) iron(II) perchlorate
967
views
Textbook Question
Give the chemical formula for each of the following ionic compounds: (e) cobalt(II) hydrogen carbonate (f) chromium(III) acetate (g) potassium dichromate.
727
views
Textbook Question
Give the name or chemical formula, as appropriate, for each of the following acids: (c) H3PO4
569
views
Textbook Question
Give the name or chemical formula, as appropriate, for each of the following acids: (d) hypochlorous acid (e) iodic acid (f) sulfurous acid.
507
views
Textbook Question
Provide the name or chemical formula, as appropriate, for each of the following acids: (a) hydroiodic acid (b) chloric acid (c) nitrous acid
569
views
