Textbook Question
Predict the sign of the entropy change of the system for each of the following reactions: (a) N2(g) + 3 H2(g) → 2 NH3(g)
663
views

 Verified step by step guidance
Verified step by step guidance

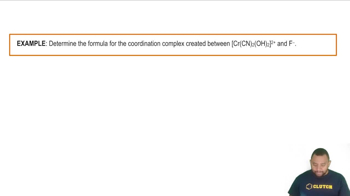

Predict the sign of the entropy change of the system for each of the following reactions: (a) N2(g) + 3 H2(g) → 2 NH3(g)
Predict the sign of the entropy change of the system for each of the following reactions: (b) CaCO3(s) → CaO(s) + CO2(g)
Predict the sign of the entropy change of the system for each of the following reactions: (d) Al2O3(s) + 3 H2(g) → 2 Al(s) + 3 H2O(g)
Predict the sign of ΔSsys for each of the following processes: (a) Molten gold solidifies.
Predict the sign of ΔSsys for each of the following processes: (b) Gaseous Cl2 dissociates in the stratosphere to form gaseous Cl atoms.