Consider the following three reactions: (i) Ti(s) + 2 Cl2(g) → TiCl4(1g) (ii) C2H6(g) + 7 Cl2(g) → 2 CCl4(g) + 6 HCl(g) (iii) BaO(s) + CO2(g) → BaCO3(s) (c) For each of the reactions, predict the manner in which the change in free energy varies with an increase in temperature.
(b) Based on your general chemical knowledge, predict which of these reactions will have K>1. (i) 2 Mg(s) + O2 (g) ⇌ 2 MgO(s) (ii) 2 KI(s) ⇌ 2 K(g) + I2(g) (iii) Na2(g) ⇌ 2 Na(g) (iv) 2 V2O5(s) ⇌ 4 V(s) + 5 O2(g)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
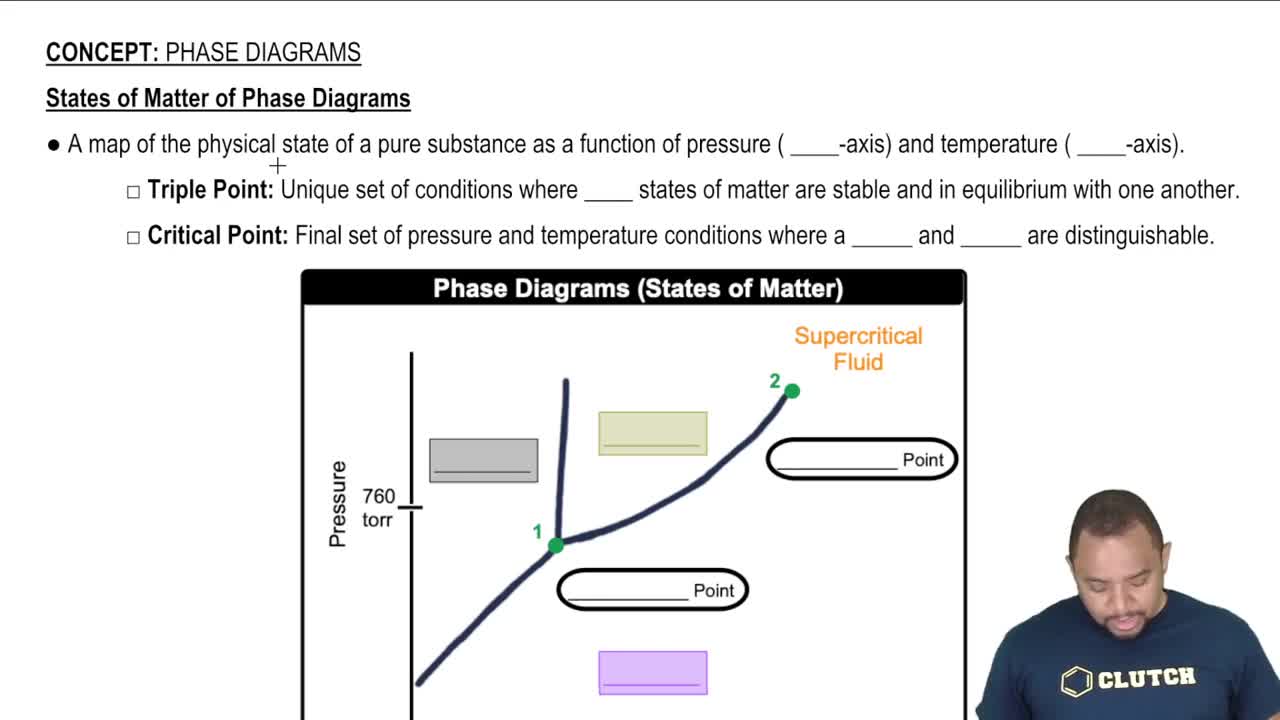
Key Concepts
Equilibrium Constant (K)

Gibbs Free Energy (ΔG)

Phase States and Their Influence on K
Using the data in Appendix C and given the pressures listed, calculate Kp and ΔG for each of the following reactions: (c) N2H4(g) → N2(g) + 2 H2(g) PN2H4 = 0.5 atm, PN2 = 1.5 atm, PH2 = 2.5 atm
(a) For each of the following reactions, predict the sign of ΔH° and ΔS° without doing any calculations. (i) 2 Mg(s) + O2 (g) ⇌ 2 MgO(s) (ii) 2 KI(s) ⇌ 2 K(g) + I2(g) (iii) Na2(g) ⇌ 2 Na(g) (iv) 2 V2O5(s) ⇌ 4 V(s) + 5 O2(g)
The conversion of natural gas, which is mostly methane, into products that contain two or more carbon atoms, such as ethane (C2H6), is a very important industrial chemical process. In principle, methane can be converted into ethane and hydrogen: 2 CH4(g) → C2H6(g) + H2(g) In practice, this reaction is carried out in the presence of oxygen: 2 CH4(g) + 12 O2(g) → C2H6(g) + H2O(g) (b) Is the difference in ΔG° for the two reactions due primarily to the enthalpy term (ΔH) or the entropy term (-TΔS)?
The potassium-ion concentration in blood plasma is about 5.0⨉10-3 M, whereas the concentration in muscle-cell fluid is much greater (0.15 M ). The plasma and intracellular fluid are separated by the cell membrane, which we assume is permeable only to K+. (a) What is ΔG for the transfer of 1 mol of K+ from blood plasma to the cellular fluid at body temperature 37 °C? (b) What is the minimum amount of work that must be used to transfer this K+?
At what temperatures is the following reaction, the reduction of magnetite by graphite to elemental iron, spontaneous? Fe3O4(s) + 2 C(s, graphite) → 2 CO2(g) + 3 Fe(s)
