You have to prepare a pH = 3.50 buffer, and you have the following 0.10 M solutions available: HCOOH, CH3COOH, H3PO4, HCOONa, CH3COONa, and NaH2PO4. Which solutions would you use?
The accompanying graph shows the titration curves for two monoprotic acids. (d) Estimate the pKa of the weak acid.

 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Titration Curves

pKa and Acid Strength
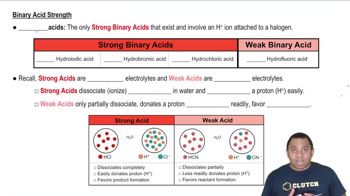
Monoprotic Acids

You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. Which solutions would you use?
You have to prepare a pH = 5.00 buffer, and you have the following 0.10 M solutions available: HCOOH, HCOONa, CH3COOH, CH3COONa, HCN, and NaCN. How many milliliters of each solution would you use to make approximately 1 L of the buffer?
Compare the titration of a strong, monoprotic acid with a strong base to the titration of a weak, monoprotic acid with a strong base. Assume the strong and weak acid solutions initially have the same concentrations. Indicate whether the following statements are true or false. (a) More base is required to reach the equivalence point for the strong acid than the weak acid.
The samples of nitric and acetic acids shown here are both titrated with a 0.100 M solution of NaOH(aq).
Determine whether each of the following statements concerning these titrations is true or false. (a) A larger volume of NaOH1aq2 is needed to reach the equivalence point in the titration of HNO3.
Determine whether each of the following statements concerning the titrations in Problem 17.35 is true or false. b. Both titration curves will be essentially the same after passing the equivalence point.
