Consider a beaker containing a saturated solution of CaF2 in equilibrium with undissolved CaF21s2. Solid CaCl2 is then added to the solution. (a) Will the amount of solid CaF2 at the bottom of the beaker increase, decrease, or remain the same?
Ch.17 - Additional Aspects of Aqueous Equilibria
Chapter 17, Problem 62b
Calculate the molar solubility of Ni(OH)2 when buffered at pH (b) 10.0.
 Verified step by step guidance
Verified step by step guidance1
Identify the dissolution equation for Ni(OH)_2: Ni(OH)_2(s) \rightleftharpoons Ni^{2+}(aq) + 2OH^{-}(aq).
Write the expression for the solubility product constant (K_{sp}) for Ni(OH)_2: K_{sp} = [Ni^{2+}][OH^{-}]^2.
Determine the concentration of OH^{-} ions from the given pH: Use the relation pOH = 14 - pH to find pOH, then calculate [OH^{-}] = 10^{-pOH}.
Substitute the [OH^{-}] value into the K_{sp} expression and solve for [Ni^{2+}], which represents the molar solubility of Ni(OH)_2.
Use the known K_{sp} value for Ni(OH)_2 to calculate the molar solubility by substituting the [OH^{-}] concentration and solving for [Ni^{2+}].

Verified Solution
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Solubility
Molar solubility refers to the maximum amount of a solute that can dissolve in a given volume of solvent at a specific temperature, expressed in moles per liter (mol/L). It is a crucial concept in understanding how substances interact in solution, particularly for sparingly soluble compounds like Ni(OH)2. The molar solubility can be influenced by factors such as pH, temperature, and the presence of other ions in solution.
Recommended video:
Guided course

Molar Solubility Example
pH and its Effect on Solubility
pH is a measure of the acidity or basicity of a solution, which can significantly affect the solubility of certain compounds. For nickel(II) hydroxide, Ni(OH)2, an increase in pH (making the solution more basic) can enhance its solubility due to the formation of soluble nickel complexes. Understanding how pH influences the dissociation of hydroxides is essential for calculating molar solubility in buffered solutions.
Recommended video:
Guided course
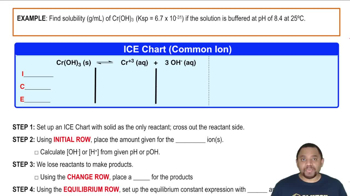
Solubility at Buffered pH Example
Equilibrium and Ksp
The solubility product constant (Ksp) is an equilibrium constant that applies to the dissolution of sparingly soluble ionic compounds. For Ni(OH)2, the Ksp expression relates the concentrations of the ions in solution at equilibrium. By knowing the Ksp value and the pH of the solution, one can derive the molar solubility by setting up an equilibrium expression that accounts for the concentration of hydroxide ions, which are influenced by the pH.
Recommended video:
Guided course

Ksp Calculations
Related Practice
Textbook Question
1186
views
Textbook Question
Calculate the solubility of Mn1OH22 in grams per liter when buffered at pH (b) 9.5.
600
views
Textbook Question
Calculate the molar solubility of Ni(OH)2 when buffered at pH (a) 8.0.
741
views
Textbook Question
Calculate the molar solubility of Ni(OH)2 when buffered at pH (c) 12.0.
329
views
Textbook Question
For each of the following slightly soluble salts, write the net ionic equation, if any, for reaction with a strong acid: (d) Hg2C2O4.
917
views
Textbook Question
From the value of Kf listed in Table 17.1, calculate the concentration of Ni2 +1aq2 and Ni1NH326 2+ that are present at equilibrium after dissolving 1.25 g NiCl2 in 100.0 mL of 0.20 M NH31aq2.
887
views
