The hypochlorite ion, ClO-, acts as a weak base. (a) Is ClO- a stronger or weaker base than hydroxylamine?
Write the chemical equation and the Kb expression for the reaction of each of the following bases with water: (c) benzoate ion, C6H5CO2-
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Base Ionization
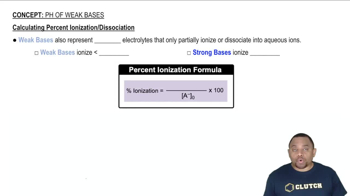
Chemical Equations

Equilibrium Constant (Kb)

The hypochlorite ion, ClO-, acts as a weak base. (b) When ClO- acts as a base, which atom, Cl or O, acts as the proton acceptor? (c) Can you use formal charges to rationalize your answer to part (b)?
Write the chemical equation and the Kb expression for the reaction of each of the following bases with water: (a) propylamine, C3H7NH2
Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base: C10H15ON1aq2 + H2O1l2 Δ C10H15ONH+1aq2 + OH-1aq2 A 0.035 M solution of ephedrine has a pH of 11.33. (a) What are the equilibrium concentrations of C10H15ON, C10H15ONH+, and OH-?
Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base: C10H15ON1aq2 + H2O1l2 Δ C10H15ONH+1aq2 + OH-1aq2 A 0.035 M solution of ephedrine has a pH of 11.33. (b) Calculate Kb for ephedrine.
Codeine 1C18H21NO32 is a weak organic base. A 5.0 * 10-3M solution of codeine has a pH of 9.95. Calculate the value of Kb for this substance. What is the pKb for this base?
