Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base: C10H15ON1aq2 + H2O1l2 Δ C10H15ONH+1aq2 + OH-1aq2 A 0.035 M solution of ephedrine has a pH of 11.33. (a) What are the equilibrium concentrations of C10H15ON, C10H15ONH+, and OH-?
Ch.16 - Acid-Base Equilibria
Chapter 16, Problem 80
Use the acid-dissociation constants in Table 16.3 to arrange these oxyanions from strongest base to weakest: SO42-, CO32-, SO32-, and PO43-.
 Verified step by step guidance
Verified step by step guidance1
Identify the conjugate acids of each oxyanion: HSO4- for SO42-, HCO3- for CO32-, HSO3- for SO32-, and HPO4^2- for PO43-.
Refer to Table 16.3 to find the acid-dissociation constants (Ka) for each conjugate acid. Remember, a lower Ka value indicates a weaker acid.
Understand that the strength of the base (oxyanion) is inversely related to the strength of its conjugate acid. Thus, the weaker the conjugate acid, the stronger the base.
Arrange the conjugate acids in order of increasing Ka values. This order will be the reverse for the oxyanions, from strongest base to weakest base.
List the oxyanions in the order derived from the previous step, starting with the one whose conjugate acid has the lowest Ka (strongest base) to the one with the highest Ka (weakest base).

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Dissociation Constants (Ka)
Acid-dissociation constants (Ka) quantify the strength of an acid in solution, indicating how well it donates protons (H+) to water. A higher Ka value corresponds to a stronger acid, which means it dissociates more completely in solution. Understanding Ka is essential for comparing the basicity of oxyanions, as the conjugate bases of stronger acids are weaker bases.
Recommended video:
Guided course

Characteristics of Ka and Kb
Oxyanions and Their Basicity
Oxyanions are negatively charged ions that contain oxygen, and their basicity is influenced by the central atom and the number of oxygen atoms. Generally, the more electronegative the central atom or the more oxygen atoms present, the weaker the base. This is due to the stabilization of the negative charge by resonance and the ability of the central atom to withdraw electron density.
Recommended video:
Guided course
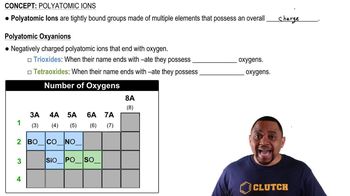
Polyatomic Oxyanions
Comparative Analysis of Oxyanions
To arrange oxyanions from strongest to weakest base, one must compare their acid-dissociation constants and the stability of their conjugate acids. By analyzing the Ka values of the corresponding acids, one can infer the relative strength of the oxyanions as bases. This comparative analysis is crucial for determining the order of basicity among the given oxyanions.
Recommended video:
Guided course

Dimensional Analysis
Related Practice
Textbook Question
1229
views
Textbook Question
Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base: C10H15ON1aq2 + H2O1l2 Δ C10H15ONH+1aq2 + OH-1aq2 A 0.035 M solution of ephedrine has a pH of 11.33. (b) Calculate Kb for ephedrine.
460
views
Textbook Question
Codeine 1C18H21NO32 is a weak organic base. A 5.0 * 10-3M solution of codeine has a pH of 9.95. Calculate the value of Kb for this substance. What is the pKb for this base?
2159
views
3
rank
Textbook Question
Given that Ka for acetic acid is 1.8 * 10-5 and that for hypochlorous acid is 3.0 * 10-8, which is the stronger acid?
1310
views
Textbook Question
Which is the stronger base, the acetate ion or the hypochlorite ion?
524
views
Textbook Question
Calculate Kb values for CH3COO- and ClO-.
1705
views
