Indicate the type of solute–solvent interaction that should be most important in each of the following solutions: c. KBr in water,
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (b) CH2Cl2 in benzene (C6H6)
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Solute-Solvent Interactions

Polarity

Ranking of Interactions
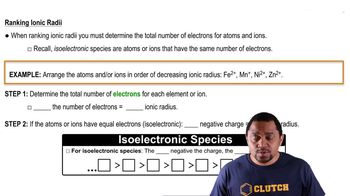
Indicate the type of solute–solvent interaction that should be most important in each of the following solutions: d. HCl in acetonitrile (CH3CN).
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (a) KCl in water
Indicate the principal type of solute–solvent interaction in each of the following solutions and rank the solutions from weakest to strongest solute–solvent interaction: (c) methanol (CH3OH) in water
An ionic compound has a very negative ∆Hsoln in water (b) Which term would you expect to be the largest negative number: ∆Hsolvent, ∆Hsolute, or ∆Hmix?
When ammonium chloride dissolves in water, the solution becomes colder. (a) Is the solution process exothermic or endothermic?
