The density of toluene (C7H8) is 0.867 g/mL, and the density of thiophene (C4H4S) is 1.065 g/mL. A solution is made by dissolving 8.10 g of thiophene in 250.0 mL of toluene. (a) Calculate the mole fraction of thiophene in the solution.
Ch.13 - Properties of Solutions
Chapter 13, Problem 51c
Calculate the number of moles of solute present in each of the following aqueous solutions: (c) 124.0 g of a solution that is 6.45% glucose (C6H12O6) by mass.
 Verified step by step guidance
Verified step by step guidance1
Identify the mass percentage of glucose in the solution, which is given as 6.45%.
Calculate the mass of glucose in the solution by multiplying the total mass of the solution (124.0 g) by the mass percentage (6.45%) expressed as a decimal.
Determine the molar mass of glucose (C_6H_12O_6) by adding the atomic masses of all the atoms in the molecular formula: 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
Use the mass of glucose obtained in step 2 and the molar mass from step 3 to calculate the number of moles of glucose using the formula: \( \text{moles} = \frac{\text{mass of glucose}}{\text{molar mass of glucose}} \).
The result from step 4 will give you the number of moles of glucose present in the solution.

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Percent by Mass
Percent by mass is a way to express the concentration of a solution, indicating the mass of solute present in a given mass of solution. In this case, a 6.45% glucose solution means that there are 6.45 grams of glucose for every 100 grams of solution. This concept is essential for determining the mass of solute in a specific mass of solution.
Recommended video:
Guided course

Mass Percent Calculation
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). For glucose (C6H12O6), the molar mass is approximately 180.18 g/mol. Knowing the molar mass allows us to convert between grams of solute and moles, which is crucial for calculating the number of moles in a solution.
Recommended video:
Guided course

Molar Mass Concept
Moles of Solute
The number of moles of solute can be calculated using the formula: moles = mass (g) / molar mass (g/mol). This relationship is fundamental in stoichiometry and solution chemistry, as it allows us to quantify the amount of solute present in a solution, which is necessary for various calculations in chemistry.
Recommended video:
Guided course
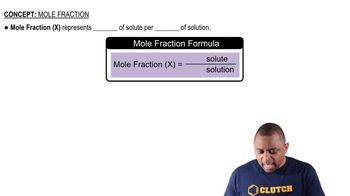
Mole Fraction Formula
Related Practice
Textbook Question
592
views
Textbook Question
The density of toluene (C7H8) is 0.867 g/mL, and the density of thiophene (C4H4S) is 1.065 g/mL. A solution is made by dissolving 8.10 g of thiophene in 250.0 mL of toluene. (b) Calculate the molality of thiophene in the solution.
1763
views
Textbook Question
Calculate the number of moles of solute present in each of the following aqueous solutions: (b) 86.4 g of 0.180 m KCl,
953
views
Textbook Question
Calculate the number of moles of solute present in each of the following solutions: (a) 255 mL of 1.50 M HNO3(aq),
1422
views
Textbook Question
Describe how you would prepare each of the following aqueous solutions from water and solid KBr: b. 125 g of 0.180 m KBr.
Textbook Question
Describe how you would prepare each of the following aqueous solutions from water and the solid solute: a. 1.50 L of 0.110 M (NH4)2SO4 solution;
